Diversity Supplements are additional financial support provided to Principle Investigators (PIs) who have already been awarded a research grant from the National Institutes of Health (NIH). The goal of these supplements is to enhance the diversity of the research workforce by financially supporting the stipends/salaries of students, postdoctorates, and eligible investigators from diverse backgrounds, including those from groups that have been shown to be underrepresented in health-related research. The research projects proposed for Diversity Supplement candidates must support work within the scope of the original project proposed in the "parent" grant.
The Funding Opportunity Annoucement (FOA) for Diversity Supplements is PA-23-189, entitled "Research Supplements to Promote Diversity in Health-Related Research (Admin Supp - Clinical Trial Not Allowed)."
Please read below to learn more about the eligible research grants that can support Diversity Supplements, the application process, and other information.
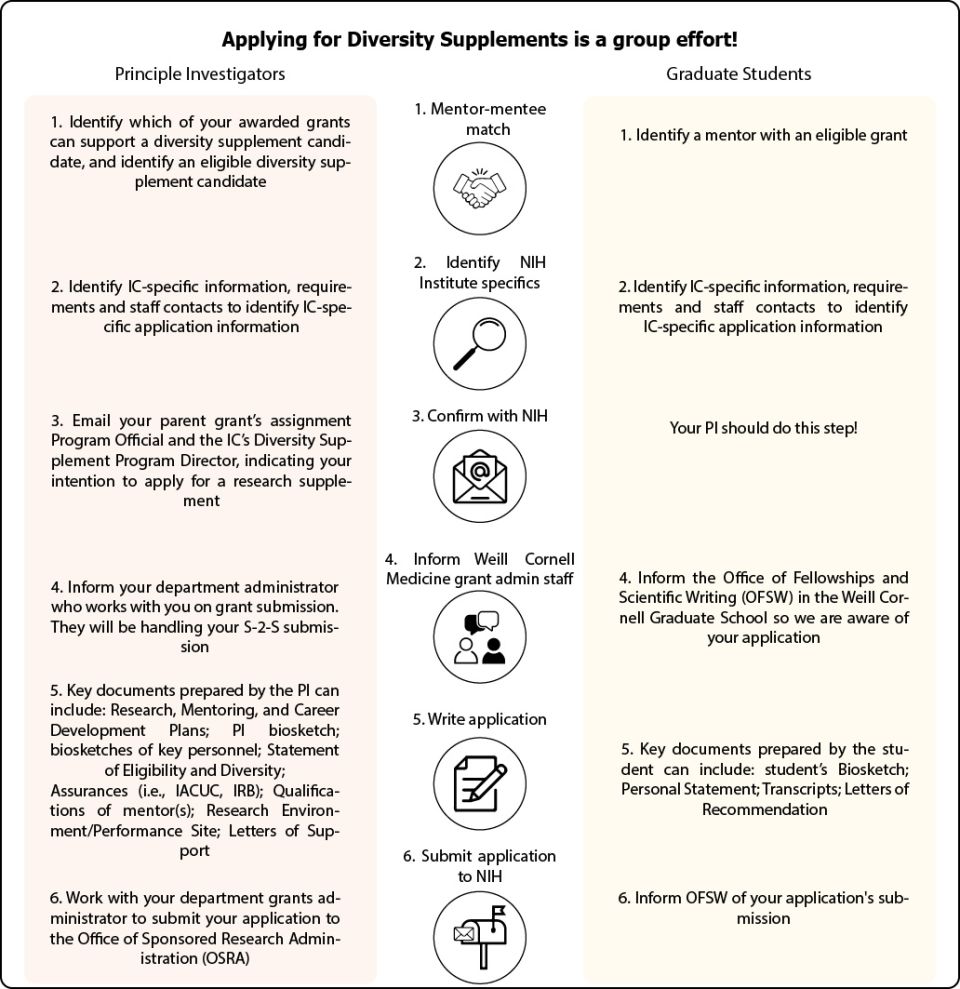
Diversity Supplement Application Process
What are the eligibility criteria for predoctoral students supported by Diversity Supplements?
1. The candidate is in the lab of Priniciple Investigator with an eligible grant from the NIH.
- Eligible grants must come from the Institutes or Centers (ICs) included on the FOA PA-23-189 (see below)
- Eligible grants must be an Activity Code included on the PA-23-189 (see below)
- Eligible grants must be funded for the entire duration of the period of ptiential funding
2. The candidate belongs to a group that the NIH considers underrepresented in the biomedical sciences, described in NOT-OD-20-031:
- Individuals from racial and ethnic groups that have been shown by the National Science Foundation to be underrepresented in health-related sciences on a national basis: Blacks or African Americans, Hispanics or Latinos, American Indians or Alaska Natives, Native Hawaiians and other Pacific Islanders
- Individuals with disabilities, who are defined as those with a physical or mental impairment that substantially limits one or more major life activities
- Individuals from disadvantaged and low socioeconomic status backgrounds
3. The candidate is not currently supported by funding from an agency of the Public Health Service (PHS)
- NRSA support from a T32, F31, or F32 cannot be terminated early to begin Diversity Supplement funding.
- Candidates currently supported by a grant (e.g., R01, R21, DP2, etc) cannot apply for a Diversity Supplement for that grant
What grants are eligible to support Diversity Supplements?
- DP1 NIH Director’s Pioneer Award (NDPA)
- DP2 NIH Director’s New Innovator Awards
- DP4 NIH Director’s Pathfinder Award- Multi-Yr Funding
- DP5 Early Independence Award
- G12 Research Centers in Minority Institutions Award
- G20 Grants for Repair, Renovation and Modernization of Existing Research Facilities
- P01 Research Program Projects
- P20 Exploratory Grants
- P2C Resource-Related Research Multi-Component Projects and Centers
- P30 Center Core Grants
- P40 Animal (Mammalian and Nonmammalian) Model, and Animal and Biological Material Resource Grants
- P41 Biotechnology Resource Grants
- P50 Specialized Center
- P51 Primate Research Center Grants
- P60 Comprehensive Center
- PM1 Program Project or Center with Complex Structure
- PN2 Research Development Center
- R00 Research Transition Award
- R01 Research Project Grant
- R03 Small Grant Program
- R15 Academic Research Enhancement Award (AREA)
- R16 Research Excellence Award
- R18 Research Demonstration and Disseminations Projects
- R21 Exploratory/Developmental Research Grant Award
- R21/R33 Phased Innovation Award
- R24 Resource-Related Research Projects
- R33 Exploratory/Developmental Grants Phase II
- R34 Clinical Trial Planning Grant Program
- R35 Outstanding Investigator Award
- R37 Method to Extend Research in Time (MERIT) Award
- R41 Small Business Technology Transfer (STTR) Grant - Phase I only
- R41/R42 Small Business Technology Transfer (STTR) Grant - Phase I, Phase II, and Fast-Track
- R41/R42 Small Business Technology Transfer (STTR) Grant - Phase I and Phase II
- R42 Small Business Technology Transfer (STTR) Grant - Phase II only
- R43 Small Business Innovation Research (SBIR) Grant - Phase I only
- R43/R44 Small Business Innovation Research (SBIR) Grant - Phase I, Phase II, and Fast-Track
- R43/R44 Small Business Innovation Research (SBIR) Grant - Phase I and Phase II
- R44 Small Business Innovation Research (SBIR) Grant - Phase II only
- R61/R33 Exploratory/Developmental Phased Award
- RC1 NIH Challenge Grants and Partnerships Program – Phase I
- RC2 High Impact Research and Research Infrastructure Programs
- RC3 Biomedical Research, Development, and Growth to Spur the Acceleration of New Technologies (BRDG-SPAN) Program
- RC4 High Impact Research and Research Infrastructure Programs – Multi-Yr Funding
- RF1 Multi-Year Funded Research Project
- RM1 Research Project with Complex Structure
- SC1 Research-Enhancement Award
- SC2 Pilot Research Project
- SC3 Research Continuance Award
- S06 Minority Biomedical Research Support Cooperative Agreements
- UL1 Linked Specialized Center Cooperative Agreement
- U01 Research Project – Cooperative Agreements
- U10 Cooperative Clinical Research – Cooperative Agreements
- U13 Conferences Cooperative Agreements
- U18 Research Demonstration – Cooperative Agreements
- U19 Research Program – Cooperative Agreements
- U24 Resource-Related Research Projects – Cooperative Agreements
- U34 Clinical Planning Grant Cooperative Agreement
- U2C Resource-Related Research Multi-Component Projects & Centers Cooperative Agreements
- U41 Biotechnology Resource Cooperative Agreements
- U42 Animal (Mammalian and Nonmammalian) Model, and Animal and Biological Materials Resource Cooperative Agreements
- U44 Small Business Innovation Research (SBIR) Cooperative Agreements - Phase II
- U54 Specialized Center- Cooperative Agreements
- U56 Exploratory Grants – Cooperative Agreements
- UC2 High Impact Research and Research Infrastructure Cooperative Agreement Programs
- UC4 High Impact Research and Research Infrastructure - Cooperative Agreement Programs
- UF1 Multi-Year Funded Research Project Cooperative Agreement
- UG1 Clinical Research Cooperative Agreements - Single Project
- UG3/UH3 Exploratory/Developmental Phased Award Cooperative Agreement
- UH2 Exploratory/Developmental Cooperative Agreement Phase I
- UH2/UH3 Phase Innovation Awards Cooperative Agreement
- UH3 Exploratory/Developmental Cooperative Agreement Phase II
- UM1 Multi-Component Research Project Cooperative Agreements
- UM2 Program Project or Center with Complex Structure Cooperative Agreement
What Institutes and Centers (ICs) participate in PA-23-189? What are IC-specific relevant deadlines?
Deadlines listed below were updated on 5/16/2023. Always check IC websites and with your NIH officials for the most up-to-date information regarding deadlines.
- National Cancer Institute (NCI). Deadlines December 1 and March 30
- National Eye Institute (NEI)
- National Heart, Lung, and Blood Institute (NHLBI) Deadlines Aug 31, Sept 30, Oct 31, Nov 30, Dec 31, Jan 31, Feb 28, Mar 3, April 30, May 31
- National Human Genome Research Institute (NHGRI) Applications accepted on continuous basis. Applications submitted after May 15 will be considered in the next fiscal year, i.e., Oct 1
- National Institute on Aging (NIA) Applications accepted on continuous basis. Applications submitted after May 1 will be considered in the next fiscal year, i.e., Oct 1
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Deadlines November 1 and May 1
- National Institute of Allergy and Infectious Diseases (NIAID) Deadlines Oct 1, Jan 1, March 1, April 1
- National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Applications are accepted year round except from June 1 through October 1
- National Institute of Biomedical Imaging and Bioengineering (NIBIB) Applications accepted on continuous basis. Applications submitted after May 31 will be considered in the next fiscal year, i.e., Oct 1
- Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Deadlines Sept 15, Jan 15, May 15
- National Institute on Deafness and Other Communication Disorders (NIDCD) Applications accepted on continuous basis.
- National Institute of Dental and Craniofacial Research (NIDCR) Applications accepted on continuous basis. Applications submitted after July 16 will be considered in the next fiscal year, i.e., Oct 1
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Deadlines Nov 1, Mar 1, Jun 1
- National Institute on Drug Abuse (NIDA) Applications accepted on continuous basis. Applications submitted after May 9 will be considered in the next fiscal year, i.e., Oct 1
- National Institute of Environmental Health Sciences (NIEHS) Deadlines Sept 1, Dec 1, Apri 1
- National Institute of General Medical Sciences (NIGMS) Applications accepted on continuous basis. Applications submitted after June may be considered in the next fiscal year, i.e., Oct 1
- National Institute of Mental Health (NIMH) Applications accepted on continuous basis. Applications submitted after April 1 will be considered in the next fiscal year, i.e., Oct 1
- National Institute of Neurological Disorders and Stroke (NINDS) Deadlines Feb 15, May 15, Nov 15
- National Institute of Nursing Research (NINR) Deadlines Jan 15, Apr 15, and Aug 15
- National Institute on Minority Health and Health Disparities (NIMHD) Deadlines Nov 1, Feb 1, June 1
- National Library of Medicine (NLM)
- Fogarty International Center (FIC) Deadline of June 29, 2023
- National Center for Complementary and Integrative Health (NCCIH) Deadlines Jan 2, Apr 1, July 1, and Oct 1
- National Center for Advancing Translational Sciences (NCATS) Deadline Sept 15
- National Institute of Environmental Health Sciences (NIEHS)
- Division of Program Coordination, Planning and Strategic Initiatives, Office of Research Infrastructure Programs (ORIP)
- Division of Program Coordination, Planning and Strategic Initiatives, Sexual & Gender Minority Research Office (SGMRO)
- Office of Strategic Coordination (Common Fund)
Institute and Center (IC)-specific contact information for PA-23-189
You can find up to date Institute and Center (IC)-specific contact information for PA-21-071 at this website: https://grants.nih.gov/grants/guide/contacts/Diversity-Supp_contacts.html
When is a good time to apply for a Diversity Supplement as a WCGS graduate student?
When you have joined your PhD lab. To apply for a Diversity Supplement as a predoctoral student, your first need to have selected your PhD advisor. Therefore, keep Diversity Supplement funding in the back of your mind as your complete your first year rotations. After you select your PhD advisor and lab, bring up your eligibility for Diversity Supplement funding to your PI.
As a WCGS graduate student, your stipend and fees are paid by the graduate school for your first two years. Starting in July at your second to third year transition, you PhD advisor starts to pay for your stipend and fees. Your second year is a great time to work your PI to apply for a Diversity Supplement.
If you are appointed to an NIH training grant or other NIH-supported program (e.g., the IMSD program), you cannot be funded by a Diversity Supplement until that appointment is complete.
Are graduate student recipients of Diversity Supplements eligible for the WCGS Fellowship Award?
Yes! The Graduate School strongly encourages eligible mentor-mentee pairs to apply for Diversity Supplements. In recognition of this support, we provide graduate student Diversity Supplement awardees with our Fellowship Award each year of their appointment.
Resources for graduate students applying for Diversity Supplements
Identifying PIs with funding eligible for a diversity supplement
Students are encouraged to use the NIH RePORTER database to confirm the NIH funding of potential mentors.
Writing the supplement
Students and PIs can receive line edits from the Office of Fellowships and Scientific Writing (OFSW). The OFSW can also work with the Office of Sponsored Research Administration (OSRA) to obtain a letter of eligiblity for diversity supplement applications.
Biosketches
When creating your biosketch, you must adhere to the current formatting guidelines, described here. SciENcv is great tool for creating your biosketch.
More questions?
If your question is not answered above, please get in touch with Dr. Nora McCall in the Office of Fellowships and Scientific Writing at nom4007@med.cornell.edu.
