The research, published Aug. 18 in Nature, focused on the effect of a high-fructose diet on villi, the thin, hairlike structures that line the inside of the small intestine. Villi expand the surface area of the gut and help the body to absorb nutrients, including dietary fats, from food as it passes through the digestive tract. The study found that mice that were fed diets that included fructose had villi that were 25 percent to 40 percent longer than those of mice that were not fed fructose. Additionally, the increase in villus length was associated with increased nutrient absorption, weight gain and fat accumulation in the animals.
Research Suggests How Tumors Evolve to Become Aggressive Form of Prostate Cancer

The genetic changes that underlie an especially lethal type of prostate cancer have been revealed in a new study by investigators at Weill Cornell Medicine. Learning more about what causes this type of cancer, called neuroendocrine prostate cancer (NEPC), could lead to new approaches for treating it.
Most early-stage prostate cancers require male hormones (androgens) like testosterone to grow. However, as they advance, they may evolve into castration-resistant prostate cancer (CRPC), a type that can grow without hormones and is harder to treat. NEPC is one type of CRPC. The findings, published June 7 in Nature Communications, revealed important findings about the basic biological actions that lead to this cancer. Dr. Nicholas Brady, an instructor in Pathology and Laboratory Medicine, and Dr. Alyssa Bagadion, a Weill Cornell Graduate School doctoral student in the Rickman lab at the time of the study, are co-first authors on the study.
“Prostate cancer is a hormone-based cancer, so many men are given hormone therapy to block the signaling that drives the cancer,” said senior author Dr. David S. Rickman, an associate professor of research in the Department of Pathology and Laboratory Medicine and a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. “However, some prostate cancers figure out how to lose their androgen receptor. They shed their dependence on androgen signaling and turn on molecular programming that allows them to grow and metastasize in the presence of androgen receptor inhibition. NEPC is particularly good at this.”
Categories: Faculty, Publications
New Super-resolution Microscopy Method Approaches the Atomic Scale
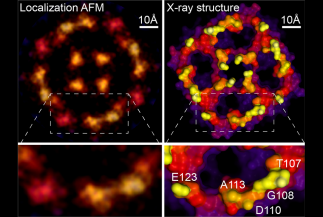
Scientists at Weill Cornell Medicine have developed a computational technique that greatly increases the resolution of atomic force microscopy, a specialized type of microscope that “feels” the atoms at a surface. The method reveals atomic-level details on proteins and other biological structures under normal physiological conditions, opening a new window on cell biology, virology and other microscopic processes.
In a study, published June 16 in Nature, the investigators describe the new technique, which is based on a strategy used to improve resolution in light microscopy.
To study proteins and other biomolecules at high resolution, investigators have long relied on two techniques: X-ray crystallography and cryo-electron microscopy. While both methods can determine molecular structures down to the resolution of individual atoms, they do so on molecules that are either scaffolded into crystals or frozen at ultra-cold temperatures, possibly altering them from their normal physiological shapes. Atomic force microscopy (AFM) can analyze biological molecules under normal physiological conditions, but the resulting images have been blurry and low resolution.
Two Investigators Win Pershing Square Sohn Prize for Cancer Research

Dr. Marcin Imielinski, an assistant professor of pathology and laboratory medicine, and Dr. Elena Piskounova, an assistant professor of cell biology in dermatology, both at Weill Cornell Medicine, were awarded the 2021 Pershing Square Sohn Prize for Young Investigators in Cancer Research on May 18.
The Pershing Square Sohn Cancer Research Alliance gives the prize annually to six early-career scientists in the greater New York City area. Each winner receives $200,000 a year for up to three years to fund innovative cancer research and encourage collaborations with industry.
“There are many impressive people in the New York scientific community who have won this award, so it’s a great feeling to be recognized,” said Dr. Imielinski, who is also a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine, and a core faculty member at the New York Genome Center. “The Pershing Square Sohn Alliance supports innovative paradigm-shifting science, so the fact that they’re willing to fund this project is very exciting. I can’t wait to get started.”
Scientists Identify Growth Signal for Metastatic Cancer "Seeds"

In 90% of cancer deaths, what kills is not the primary tumor. Rather, it’s the spreading of cancer to other vital tissues and organs, what’s called metastasis, that proves deadly.
Scientists are eager to better understand this process so they can intervene in it, potentially saving lives.
Cell biologist Joan Massagué has been systematically dissecting metastasis for more than two decades. His research has shown that it is the end result of a long process that can be divided into several steps, each with its own challenges.
The latest study to emerge from his lab identifies the final hurdle that metastatic cancer cells, like microscopic seeds, must overcome to take root and sprout somewhere else.
“We’ve identified a molecule that metastasis-initiating cancer cells need in order to start growing in a new location,” explains Dr. Massagué, who also serves as Director of the Sloan Kettering Institute. “If we deprive the cells of this molecule, they can still infiltrate distant organs, but they fail to grow into a new tumor.”
Categories: Faculty, Publications
New Imaging Technique Provides Snapshot of Brain Tumor Activity
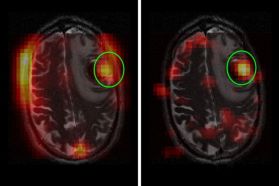
Brain tumors are challenging to treat and monitor over time. Their location in the body’s control center makes it especially urgent for doctors to know if a treatment is working or if they should switch strategies. But it is hard to get this information quickly.
Now Memorial Sloan Kettering researchers have shown that a new diagnostic technique could markedly shorten this period of uncertainty. The technology is called hyperpolarized magnetic resonance imaging (HP MRI). It could potentially allow doctors to see whether a tumor is responding to treatment within just a few days.
“This could make a dramatic impact in helping doctors treat people with primary and metastatic brain tumors more effectively,” says Kayvan Keshari, a biochemist and imaging specialist.
Categories: Faculty, Publication
Findings from Two Patients Shed New Light on Drug Resistance in AML
Last summer, the US Food and Drug Administration approved enasidenib (Idhifa®) for the treatment of acute myeloid leukemia (AML). Enasidenib works differently than most cancer drugs. Rather than killing leukemia cells, it turns them into normal blood cells. Memorial Sloan Kettering hematologist-oncologist Eytan Stein led the pivotal clinical trial that resulted in the drug’s approval.
Now, a collaborative team of researchers is reporting that people who take enasidenib can develop resistance to it — and in a way never seen before. The findings are being reported in Nature.
“Everyone who studies precision medicine spends a lot of time thinking about why some people respond to certain drugs and why some stop responding or never respond at all,” says physician-scientist Ross Levine, who was one of the paper’s senior authors, along with Dr. Stein. “MSK has been one of the leaders in figuring this out.”
The discovery was made by a team of doctors, laboratory researchers, and pharmaceutical company scientists. They used cells from people who were being treated with enasidenib to uncover why the drug sometimes stops working.
Categories: Faculty, Publications
Don't Scratch That Mole? Scientists Are Learning More about Inflammation and Cancer
Growing up, Philipp Niethammer learned from his mother to never scratch his moles.
“I am not sure I knew what might happen if I scratched,” says the Sloan Kettering Institute cell biologist. “But I knew it was bad, so I didn’t do it.”
That old warning resurfaced in Dr. Niethammer’s mind not too long ago when he read a research article describing how repeated injury to the tail fin of a zebrafish could cause tumors to develop.
Like humans, zebrafish are prone to getting melanoma, a type of skin cancer. The study found that injuring the fish’s tail fin every couple of weeks for several months greatly increased the rate of cancer formation in that spot.
Intrigued by the finding, Dr. Niethammer wrote a commentary on the paper, extrapolating what the results might mean for humans. He called it “Do not scratch that mole!”
Categories: Faculty, Publications
Out of the Closet, into the Lab: Five LGBTQ Scientists Share Their Stories

They’re here. They’re queer. They’re scientists.
In honor of Pride month, we wanted to explore what it’s like to be both a scientist and a person who identifies as lesbian, gay, bisexual, transgender, or queer.
Does being LGBTQ present unique challenges to one’s career as a scientist? Is it harder or easier to be out in science than in other fields? What difference — if any — does being queer make to a scientific life?
To find out, we spoke to five scientists at Memorial Sloan Kettering, each of whom has had very different, and illuminating, experiences.
Categories: Faculty, Publications
What Is Epigenetics, and Why Is Everyone Talking about It?

If you’ve ever known a set of identical twins, you might have been struck by what they didn’t have in common. Maybe one was a skilled musician, while the other couldn’t carry a tune. Maybe one craved adventure, while the other preferred the couch. These anecdotal examples reveal a fundamental truth about genetics: Even two people who share the exact same DNA can differ substantially in their attributes.
The same is true of cells. Every cell in your body — from the retinal cells in your eyes to the skin cells in your toes — contains the same set of about 20,000 genes. Yet somehow, this common genetic blueprint provides the basis for each cell’s individuality.
Welcome to epigenetics, the study of how these curious differences come about.
Epigenetics has captured the public’s attention in recent years in a way that few other technical scientific topics have. It’s not uncommon to read headlines about the “epigenetic revolution” and hear claims that epigenetics overthrows our understanding of evolution. So what exactly is epigenetics? How does it work? And why does it matter?
Categories: Faculty, Publications
Cancer Cells Eat Fat to Grow and Spread
Cancer cells get hungry. Very hungry.
It takes a lot of energy to reproduce the way they do — repeatedly and often. Pound for pound, fat has more energy than any other nutrient. So it’s perhaps not surprising that when cancer cells find themselves in fat tissue, they make quick use of these resources.
According to a new study from researchers at the Sloan Kettering Institute at Memorial Sloan Kettering, the presence of fat may even be what helps cancer cells take root in the first place. Richard White, a physician-scientist in the Cancer Biology and Genetics Program at SKI, discovered that melanomas preferentially grow near adipose (fat) tissue and eagerly eat up the abundant fats, also called lipids, found there.
Categories: Faculty, Publications
How the Zebrafish Got Its Stripes

If you’re a zebra, you want your stripes in order. Come to the savannah wearing spots and someone might mistake you for a leopard.
Proper pigmentation is important for a variety of animal endeavors, including finding and selecting a mate, hiding from predators, and protecting oneself from damaging UV light. But it’s even more than that.
“Pattern formation in the embryo is how every animal organizes its tissues,” says Richard White, a physician-scientist in the Cancer Biology and Genetics Program at the Sloan Kettering Institute. “That’s true of the pancreas, it’s true of the kidney, it’s true of the skin. Patterning is how animal bodies are made.”
Dr. White’s lab studies patterning in an animal called the zebrafish, a small freshwater fish with distinctive black-and-white stripes running down its length.
In a new study published in Developmental Cell, "Distant Insulin Signaling Regulates Vertebrate Pigmentation through the Sheddase Bace2", Dr. White and graduate student Yan Zhang reveal how zebrafish get their stripes. Unexpectedly, it has to do with insulin.
Categories: Faculty, Publications
Neutrophil Recruitment — What's Damage Got to Do with It?
Every second of our lives, we are assaulted by a barrage of microbes that can make us sick. That we don’t fall ill more frequently is a testament to the effectiveness of our innate immune system in deterring these enemies at the gate.
The innate immune system consists of the defenses we’re born with to fight infections. Typically, the first innate actors to respond to signs of trouble are the white blood cells called neutrophils. These skilled microbe hunters swoop into the area and begin releasing toxic chemicals while also gobbling up pathogens.
We would be lost without our neutrophils. And, indeed, when neutrophil counts drop — as can happen during cancer treatment — the risk of infection goes way up. (This low-neutrophil state is called neutropenia.)
But neutrophils don’t respond only to infections. They also swarm to damaged tissue that’s unrelated to an infection, such as during a heart attack or stroke. Rather than help in these situations of sterile tissue damage, neutrophils actually make matters worse; they cause more tissue damage and delay healing.
Categories: Faculty, Publications
Highlights from MSK at the 2018 American Association for Cancer Research Meeting

A major milestone in cancer genetics — and a topic of much discussion at the meeting — was the publication of the Pan-Cancer Atlas. Based on the analysis of 10,000 tumor samples, the atlas lays out a massive body of data. It describes the molecular changes that occur in 33 cancer types. This genetic blueprint was presented in 27 papers published in several major scientific journals in the days leading up to the AACR meeting.
MSK cancer biologist Nikolaus Schultz was a co-corresponding author of one of the most prominent papers, published in Cell. In the study, a large research team analyzed genetic data to shed light on ten signaling pathways linked to cancer. (A signaling pathway is a group of molecules in a cell that work together to control one or more cell functions, such as cell division or cell death.) They looked at genetic changes in these signaling pathways across the 33 cancer types.
Across the different cancers, they found that 89% of tumors had at least one driver alteration, a genetic change that promotes cancer and could be a good target for drugs. In addition, 57% had at least one alteration potentially targetable by currently available drugs. They also found that 30% of tumors had multiple targetable alterations, suggesting that many cancers could respond well to combination therapies.
Categories: Faculty, Publications
Scientists Identify How Gene Mutation Drives a Deadly Childhood Cancer

Researchers at the Sloan Kettering Institute have unraveled how a genetic mutation drives a type of aggressive soft tissue cancer called synovial sarcoma. This often-deadly cancer primarily affects children and young adults. It rarely responds to chemotherapy.
Using patient-derived cancer cell lines and the genome-editing tool CRISPR, the team of scientists led by molecular biologist Scott Lowe showed how the protein product of this mutation wreaks havoc: It hijacks molecules that normally turn genes off during development, turning them on instead.
“It’s an insidious mechanism that the oncogene uses to drive the cancer,” Dr. Lowe says. “But it also points us to potential treatment approaches."
The genetic mutation at the root of synovial sarcoma causes two separate genes from different chromosomes to become spliced together. The conjoined genes produce a novel “fusion” protein called SS18-SSX1/2. This fusion mutation is found in nearly all of synovial sarcoma tumors and is often the only genetic mutation identified. That suggests it plays a role as the primary driver of the cancer.
Categories: Faculty, Publications
Escape Artists: Cancer Cells Mimic Immune Cell Activity to Spread
A new finding suggests that a genetic mistake in cancer cells known as chromosomal instability creates an inflammatory state within the cells. The cancer cells take advantage of this condition by hijacking pathways used by immune cells to migrate. This enables these escape artists to slip away from the primary tumor and move to other parts of the body. The insight could guide treatment strategies to block cancer from spreading.
Study Reveals How Malaria Parasites Prepare for Transmission

The complex life cycle of the parasite that causes malaria has made it a difficult foe to beat. But new insights on how the parasite is transmitted from humans to the mosquitoes that spread malaria may lead to new ways to control this deadly disease, which in 2015 affected 212 million people worldwide and resulted in 429,000 deaths—mostly children under age 5.
In a study published Sept. 25 in Nature, a team led by Weill Cornell Medicine investigators Drs. Björn Kafsack and Olivier Elemento describe new genes that are activated once the parasite commits to transmission.
Categories: Faculty, Publications
Chen, Studer: Stem Cell Research Identifies Potential Drugs for Treating Zika Infection

Over the past few years, thousands of babies whose mothers were infected with the mosquito-borne virus Zika have been affected by a devastating birth defect called microcephaly, in which the head and brain are small and don’t develop properly. Although the virus has been known for decades, public outcry for a vaccine or treatment grew louder in 2015 and 2016 as the virus spread.
Now investigators from Memorial Sloan Kettering and Weill Cornell Medical College are using a combination of tools in the lab — including mouse models and tiny stem cell–derived, brain-like structures called organoids — to identify drugs that could either prevent Zika infection or treat ongoing infections.
Hajjar: Protein Prevents Excess Fluid from Entering Lung Tissue

Dr. Katherine Hajjar. Photo credit: Robert Essel
A protein found in the cells lining blood vessels plays a central role in preventing fluid and inflammatory cells from leaking into lung tissue in a low-oxygen environment, Weill Cornell Medicine researchers discovered. Their findings may enable the development of more effective treatments for a condition called pulmonary edema, in which excess fluid makes it difficult to breathe.
In the study, "Annexin A2 Supports Pulmonary Microvascular Integrity by Linking Vascular Endothelial Cadherin and Protein Tyrosine Phosphatases", published July 10 in The Journal of Experimental Medicine, the scientists genetically engineered mice without the protein annexin A2. They exposed rodents with and without the protein to a low-oxygen or normal-oxygen environment for 48 hours, injected an experimental dye and then examined their organs. The investigators found that mice without annexin A2 showed a two-fold greater leakage of dye into lung tissue compared to mice with the protein.
Blanchard: New Imaging Technique Allows Researchers to See Molecular Machinery at Work

New imaging methods that allow researchers to track the individual protein molecules on the surface of cells have been developed by Weill Cornell Medicine investigators. The results offer unprecedented insight into how cells sense and respond to their environments.
G protein-coupled receptors (GPCRs) are proteins that reside within the cellular membrane and relay signals into the cell to regulate fundamental aspects of human physiology. The signals received through GPCRs include everything from light, which activates the proteins in cells that enable vision, to chemicals such as neurotransmitters that regulate mood, to signals that trigger pain. Nearly half of all clinically used drugs work by targeting distinct GPCRs.
“These proteins are critical to every aspect of human physiology,” said co-senior study author Dr. Scott Blanchard, a professor of physiology and biophysics at Weill Cornell Medicine. “We need to know how GPCRs recognize all of these signals, how they process the signals and how they transmit the information into the cell to invoke a specific action. Only in doing so will we be able to develop new generations of drugs that more accurately target these proteins and thus can help without causing collateral damage.”
Evans: Researchers Develop First-Ever Human Tissue Platform to Test Drugs for Colon Cancer
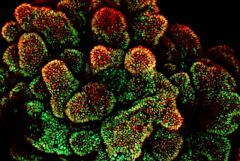
The first-ever “disease in a Petri dish” platform that models human colon cancer derived from stem cells has been developed by Weill Cornell Medicine investigators, allowing them to identify a targeted drug treatment for a common, inherited form of the disease. The discovery also overcomes a longstanding challenge of using mice to research this form of cancer, as they do not typically develop the disease.
In the study, "Colonic Organoids Derived from Human-Induced Pluripotent Stem Cells for Modeling Colorectal Cancer and Drug Testing", published June 19 in Nature Medicine, the scientists used human-induced pluripotent stem cells (iPSCs), which can in principle differentiate into any type of cell in the body, that were derived from the skin of two patients with an inherited form of colorectal disease called familial adenomatous polyposis (FAP). With FAP, large intestine cells develop into numerous polyps that for these patients eventually become colon cancer. Using iPSCs, they developed 3D structures called colonic organoids that closely represented large intestine tissue systems and then performed drug testing.
Photo: This is a projection image displaying all of the colon organoid layers. The red color represents cells that line the colon called epithelial cells and the green color represents an increase in the number of cells that are proliferating. The organoids are derived from induced pluripotent stem cells, which can differentiate into any type of cell in the body, derived from a patient with a colorectal disease called familial adenomatous polyposis (FAP). Photo credit: Dr. Miguel Crespo.
Wendel: When Loss Is a Gain: New Tumor Suppressor Gene Identified in Follicular Lymphoma

Scientists at the Sloan Kettering Institute are investigating how chromosome changes underlie the development of follicular lymphoma.
Follicular lymphoma is a slow-growing yet incurable form of non-Hodgkin lymphoma. New research is shedding light on the molecular causes of this type of cancer. Scientists have identified a gene that functions as a tumor suppressor. Loss or inactivation of this gene promotes cancer.
Highlights
- About 30% of people with follicular lymphoma are missing a portion of chromosome 6. This genetic abnormality is associated with a worse outcome.
- Scientists have identified a gene on that portion of chromosome 6 that functions as a tumor suppressor.
- Cancers missing this gene may be vulnerable to certain treatments that block the action of another cellular actor called mTOR. These drugs are being tested in clinical trials.
Hadjantonakis: It Takes Two: A Pair of Proteins Coordinate to Direct Development of Embryonic Cells
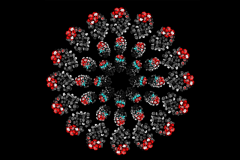
This image, generated in the lab of developmental biologist Anna-Katerina (Kat) Hadjantonakis, shows mouse embryos arranged in three circles. The embryos’ appearance varies according to which genes are switched on or off. These developmental genes direct the fate of individual cells during the earliest stage of life.
The image appears on the cover of the June 5 issue of Developmental Cell. Inside, Dr. Hadjantonakis’s team reports an important finding on how genes function during normal mammalian development. Her research, which combines the study of genetics and imaging, is essential for clarifying this process in hopes of putting it to therapeutic use. Guiding undifferentiated cells to become specific cell types could make it possible to replenish those lost to disease or injury.
Weill Cornell Medicine Team Creates Self-Renewing Hematopoietic Stem Cells for Transplantation
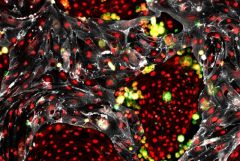
Researchers at Weill Cornell Medicine have discovered an innovative method to make an unlimited supply of healthy blood cells from the readily available cells that line blood vessels. This achievement marks the first time that any research group has generated such blood-forming stem cells.
“This is a game-changing breakthrough that brings us closer not only to treat blood disorders, but also deciphering the complex biology of stem-cell self-renewal machinery,” said senior author Dr. Shahin Rafii, director of the Ansary Stem Cell Institute, chief of the Division of Regenerative Medicine and the Arthur B. Belfer Professor at Weill Cornell Medicine.
Cesarman: Researchers Identify a Drug that Blocks Some Blood Cancers

Dr. Ethel Cesarman
A compound identified by Weill Cornell Medicine scientists inhibits the growth of a rare blood cancer found in people with HIV-AIDS. Their research, published May 15 in the Journal of Clinical Investigation, also demonstrates that the compound deters multiple myeloma, another type of blood malignancy.
Few effective treatments are available for primary effusion lymphoma (PEL), a subtype of non-Hodgkin lymphoma (NHL) that is characterized by aggressive growth of cancer in the body cavities. It accounts for 4 percent of the people diagnosed with NHL.
“There are very few cases of primary effusion lymphoma diagnosed each year, but it has a bad prognosis,” said senior study author Dr. Ethel Cesarman, a professor of pathology and laboratory medicine and a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. While patients with PEL need new treatments, the newly identified compound may one day be another approach to treating multiple myeloma, a more common condition, she said.
Dow: New Method Developed to More Accurately Model Colorectal Cancer

An engrafted tumor created by transplanting organoids into a mouse colon - photo: Kevin O’Rourke.
Researchers have long struggled to develop preclinical models of colorectal cancer in which the disease forms in the correct anatomical location. Now Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center researchers have developed such a model, which could serve as a platform for scientists to study every stage of the disease.
“I was shocked to learn that most mouse models that were considered state-of-the-art for studying colon cancer didn’t actually get cancer in their colon,” said Kevin O’Rourke, an MD-PhD student in the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD program, who works in the lab of Dr. Scott Lowe at Memorial Sloan Kettering. “Instead, benign, precancerous tumors form in the small intestine in these models, and the reason for this is still not fully understood.”
Jallepalli: New Study Shows How Wayward Chromosomes Get Back on Track
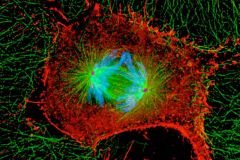
Highlights
- Studying the mechanisms of cell division gives researchers key insights into the inner workings of our cells.
- If errors occur during cell division, it can result in cancer.
- This research may lead to new therapies that disrupt the cell division process in tumors.
Rubin: Three-Pronged Approach Key to Precision Medicine
Published March 22 in Cancer Discovery, the study brings researchers one step closer to fulfilling the promise of precision medicine, which aims to provide targeted, individualized treatment based on each patient’s genetic profile.
“Our goal is to use precision medicine to improve the way clinicians think about cancer therapies as opposed to selecting a therapy that may not be fitted to that patient’s cancer,” said senior author Dr. Mark Rubin, director of the Englander Institute for Precision Medicine and the Homer T. Hirst III Professor of Oncology in Pathology at Weill Cornell Medicine, and director of the joint precision medicine program at Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center. “Combining genome sequencing with rapid drug screening enables us to nominate new therapies for patients, which we could not have predicted from the genomics alone.”
Petrini: Understanding the DNA-Damage “First Responders”: John Petrini at Work

Highlights
- Cells have molecules that directly sense damaged DNA and repair it.
- Such repair is crucial for maintaining the stability and fidelity of genetic information.
- Deficiencies in DNA repair can lead to mutations that cause cancer.
- Targeting DNA-repair pathways has promising therapeutic potential.
Huangfu: Connecting the Dots: Stem Cells Provide Valuable Tool for Linking Genes and Disease
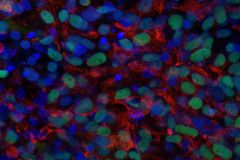
Highlights
- Pluripotent stem cells have the ability to develop into any type of cell in the body.
- By growing cells in a dish, researchers can learn how certain diseases form.
- Understanding the role that specific genes play in disease may lead to new treatments.
Sawyers: Stop, Collaborate, and Share Data: AACR Project GENIE Seeks to Advance Cancer Treatment

A collaboration among eight leading cancer centers, including MSK, is aiming to take advantage of genomic sequencing of patient tumors to advance the field of precision oncology. On January 5, the group is announcing the release of data on nearly 19,000 cancer patients.
Highlights
- The AACR Project GENIE initiative is being coordinated by the American Association for Cancer Research.
- Charles Sawyers, a physician-scientist at MSK, serves as chair of the project’s steering committee.
- The database includes genomic sequencing of patients’ tumors and follow-up clinical records.
- The data will serve as a tool for cancer researchers around the world.

