
Research
- Membrane biophysics and how it affects the Functional mechanisms of neurotransmitter transporters: We developed an unprecedented understanding of the mechanisms that drive the functions of neurotransmitter transporters in neuronal processes and mechanisms of drugs of abuse (see 1a in List of Selected Publications)
- Allostery and the mechanisms of molecular machines at the membrane. Triggering and modulation of functional mechanisms of many biomolecular systems involves mechanisms that connect distal regions of the molecules, along “allosteric pathways”. Using novel analytical and numerical formulations we identify structural microdomains that act as
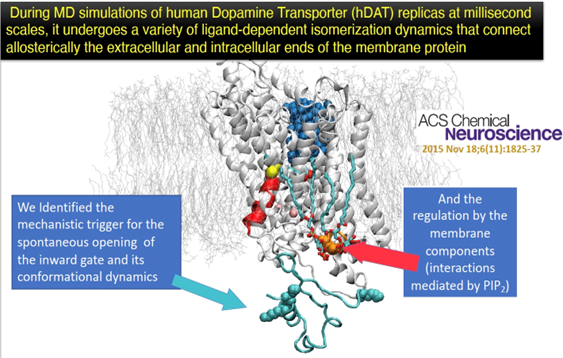
Figure: (i)-allosteric communication channels for long-distance information sharing between functional sites, and (ii)-as allosteric coordinators that organize dynamics within functional sites. We pioneered this level of inquiry that serves now to informs about mechanism, function nder diverse conditions, repair of dysfunctional mutated systems, substitution, and repurposing of cell components of molecular machines such as GPCRs, TMEM16 scramblases, and transporters of various solutes (See 2a; 2b)
- Development and implementation of novel methods for analysis of biomolecular mechanisms: We are continuously developing new approaches based on AI, ML, and molecular physics for analysis, interpretation, and quantification of results from the experimental and computational simulation assays of biomolecular function (See 3a; 3b in Selected Publications).
- Since 2020 an additional area of research is the study of the molecular mechanisms of the SARS-CoV-2 to be leveraged for the design of antiviral therapy. (See 4a;4b;4c in publications)
Bio
Weinstein Received his BSc degree in Chemistry, MSc in Quantum Chemistry, and DSc in Theoretical Physical Chemistry from the Technion, Israel Institute of Technology. His postdoctoral training was at Johns Hopkins with Robert G. Parr, after which he started his independent career as an Assistant Professor of Pharmacology at Mount Sinai School of Medicine (MSSM) in NYC, rising through the ranks to Full Professor. In 1985 he became the Dr. Harold and Golden Lamport Professor and Chairman of the Department of Physiology and Biophysics at MSSM. He was recruited in 2002 to Weill Cornell as the Maxwell Upson Professor, Chair of the Department of Physiology and Biophysics, the Founding Director of the Institute for Computational Biomedicine (ICB), and Tri-Institutional Professor. In 2021 he stepped down as department chair and maintains his other appointments and activities.
Selected Publications:
1a. *LeVine MV, Cuendet AM, Khelashvili G; Weinstein H – Allosteric Mechanisms of Molecular Machines at the Membrane: Transport by Sodium-Coupled Symporters. Chem Rev. 2016, 116:6552-6587
2a. *Xie H and Weinstein H. – Allosterically coupled conformational dynamics in solution prepare the sterol transfer protein StarD4 to release its cargo upon interaction with target membranes. Front. Mol. Biosci. 2023 eCollection 2023.
2b. Khelashvili G, Kots E, Cheng X, Levine MV, Weinstein H. – The allosteric mechanism leading to an open-groove lipid conductive state of the TMEM16F scramblase. Communications Biol. 2022 5(1):990.
3a. *Plante A and Weinstein H. – Ligand-Dependent Conformational Transitions in Molecular Dynamics Trajectories of GPCRs Revealed by a New Machine Learning Rare Event Detection Protocol. Molecules 2021, 26(10):3059.
3b. Kots E, Shore DM, Weinstein H. – Simulation of pH-Dependent Conformational Transitions in Membrane Proteins: The CLC-ec1 Cl-/H+ Antiporter. Molecules. 2021 26(22):6956
4a. Khelashvili G, *Plante A, Doktorova M, Weinstein H. – Ca2+-dependent mechanism of membrane insertion and destabilization by the SARS-CoV-2 fusion peptide. Biophys J 2021 120:1105–19
4b. Bram Y, Duan X, Nilsson-Payant BE, Chandar V, Wu H, Shore D, Fajardo A, Sinha S, Hassan N, Weinstein H*, TenOever BR*, Chen S*, Schwartz RE* – Dual-Reporter System for Real-Time Monitoring of SARS-CoV-2 Main Protease Activity in Live Cells Enables Identification of an Allosteric Inhibition Path. ACS Bio Med Chem Au 2022, 2(6):627–641.
