
Research
The vertebrate retina is a neural circuit composed of different cell types organized in a laminated structure. The cell soma and processes of different retinal cells are segregated into anatomically distinctive layers. Photoreceptors are the predominant type of retinal neurons that sense light. Retinal pigment epithelial (RPE) and Müller glial cells are the main supporting cells that have multitasking functions including removing and recycling the waste produced by the neighboring photoreceptors. The eye is also a window to the brain.
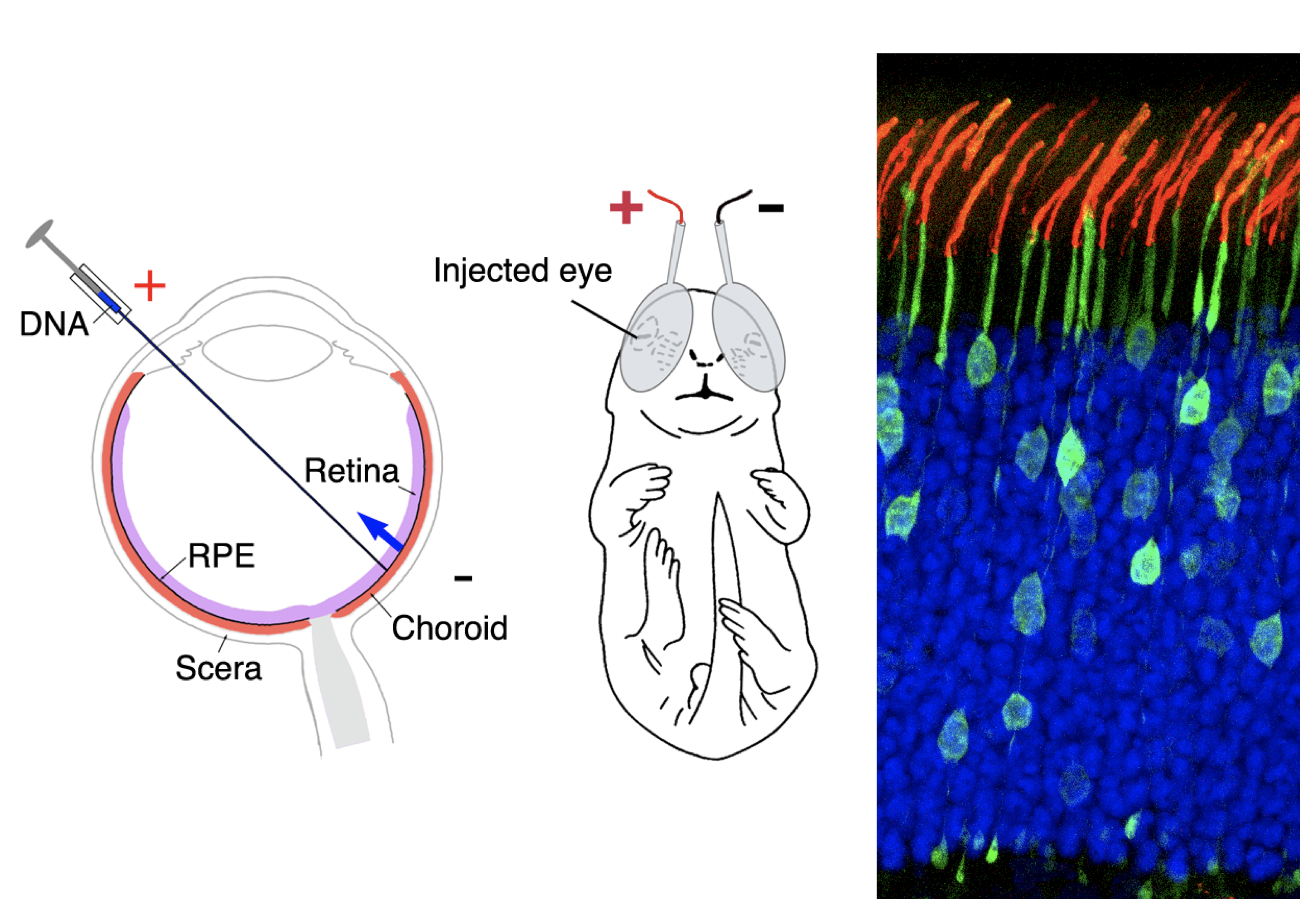
Figure 1 Retinal transfection. (left) Schematic drawing of the subretinal injection and electroporation procedures of neonatal rodents to deliver plasmids to the desired retinal cell types. This is a useful approach to complement the conventional transgenic mouse study. (Right) An image of mouse retina containing transfected rod photoreceptors (green) and stained rhodopsin (red) in the light-sensing cilia (blue) photoreceptor nuclei.
The Sung lab uses genetics, confocal, 3D scanning EM, biochemistry, cell cultures, and next-generation sequencing approaches to uncover the basic processes that maintain the specialized structure/function of photoreceptors, RPE, and glia in normal and perturbed retinas. Dr. Sung’s lab studies how defects, often caused by the mutations found in patients, lead to retinal degeneration and vision loss. To understand these complex processes, the lab has made mouse lines that feature the retinal pathophysiology of patients with retinitis pigmentosa, the most common form of inherited retinal disorder, and dry age-macular degeneration (AMD), the leading cause of blindness in older adults. These mice lines are also being used as potential tools for live testing of early diagnostic biomarkers and therapeutic candidates for ameliorating pathological lesions and their associated neuronal degeneration. The lab has devices for live mouse eye imaging, electrophysiology, and behavior tests. The eye is also a window to the brain. The lab’s recent studies with these mice also show the retina to share pathological hallmarks of other common neurological diseases and lysosomal storage diseases, making the eye an accessible surrogate to study the pathophysiology of these diseases.
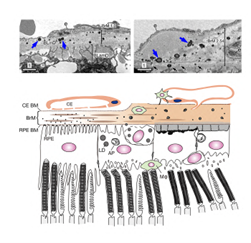
Figure 2 Dry A MD Studies. (Top) Lipid-rich deposits resembling drusen (blue arrows), which is the pathological hallmark of AMD, are observed in the conditional CLIC4 knockout mice. BrM: Bruch's membranes; BLinD: basal linear deposits; BLamD: Basal laminar deposits; e: endothelial cell. (Bottom) A cartoon depicts the retinal-RPE-Bruch’s membranes-choroid complex of normal mice (left) and the progressively developed lesions in the CLIC4 mutant mice (middle to right). CE choriocapillaris; BM basement membranes; AP autophagosomes; LD: lipid droplet: Macrophages (green). Nature Communications, 13: 374.
Current Projects:
- The endolysosomal trafficking-proteostasis coupling in retinal diseases
Bio
Sung earned her bachelor’s degree (in Biochemistry) from National Taiwan University, and PhD (in Microbiology & Immunology) from National Yang-Ming University, Taiwan. Dr. Sung received the HHMI fellowship and worked as a postdoctor associate under the mentorship of Dr. Jeremy Nathans at Johns Hopkins University School of Medicine. Sung joined WCGS in 1994. She is now a member and professor at WCGS.
Distinctions:
- Keith R. Porter Fellow for Cell Biology, ASCB (2001)
- Ruth and Milton Steinbach Macular Degeneration Research Award (2001)
- Lew R. Wasserman Merit Award, Research To Prevent Blindness (2002)
- Senior Investigator Award, Research To Prevent Blindness (2010)
- Distinguished Alumni Award, Yang-Ming National University, Taiwan (2011)
- Stein Innovation Award, Research To Prevent Blindness (2016)
- Senior Investigator Award, Alcon Research Institute (2017)
