
Research
The Niethammer lab studies the chemical, and physical mechanisms through which living tissues sense their integrity and trigger immune and repair mechanisms over large distances when integrity is lost. These mechanisms are broadly relevant for the initiation of inflammatory diseases, such as asthma and colitis, tissue regeneration, and cancer. To thoroughly dissect them, we apply an interdisciplinary mix of methods ranging from intravital imaging in zebrafish to computational modeling and in vitro reconstitution.
Our main approaches comprise:
- Genetics, lipidomics and intravital imaging in live zebrafish larvae. Zebrafish are an extremely powerful vertebrate model that faithfully resembles mammalian healing and immune defense.
- Computational image analysis and mathematical modeling of wound signaling for thorough quantitative understanding.
- Biochemical and biophysical reconstitution of molecular mechanisms in cultured cells/organoids, and artificial membrane systems.
Our current fields of interest are:
- ROS and lipid signaling during infection, colitis, skeletal homeostasis, & cancer.
- Mechanisms of nuclear membrane mechanotransduction in health and disease.
- Osmotic surveillance as physical key regulator of wound detection (Enyedi et al., NCB, 2013; Gault et al., JCB, 2014; Enyedi et al., Cell, 2016; Huang & Niethammer, Immunity, 2018).
- Reactive oxygen species as chemical key regulators of wound detection (Niethammer et al., Nature, 2009; Katikaneni et al., NCB, 2020).
- Nuclear membrane mechanotransduction (Enyedi et al., Cell, 2016; Shen et al., PNAS, 2022).
- Oxoeicosanoid signaling as novel infection defense mechanism (Ma et al., Cell Rep, 2023).
Figure 1
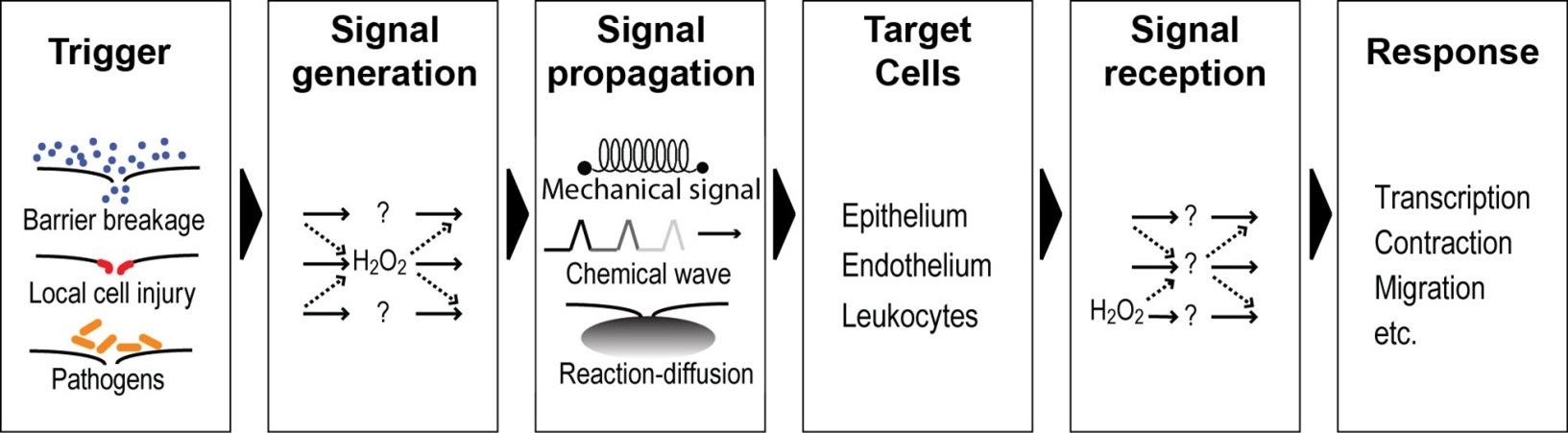
Current Projects:
- Mechanosensitive lipid signaling in skeletal homeostasis.
- Oxoeicosanoids: Synthesis and physiological/pathological functions.
- The cell biological foundations of nuclear membrane mechanotransduction.
- Wound sensing by blood vessels.
Bio
We investigate how leukocytes are rapidly targeted to sites of “danger” in intact tissues, i.e. how they detect wounds or sites of infection. Misregulation of this process may lead to hyper-inflammatory disease. Chronic inflammatory states, in turn, promote cancer. Although the complex cytokine networks that govern amplification and resolution of inflammatory responses have been intensely studied, the initial mechanisms that regulate leukocyte targeting in tissues remain very little understood.
Distinctions:
- Dorsett L. Spurgeon Distinguished Medical Research Award
- American Asthma Foundation Scholar
- Boyer Basic Research Award
