
Research
The innate immune system uses germline-encoded receptors to detect and respond to pathogens. At least six human innate immune receptors recognize specific pathogen-associated danger signals and assemble into multi-protein complexes called inflammasomes (Figure 1). Inflammasomes recruit and activate inflammatory caspases, thereby triggering a lytic form of cell death called pyroptosis. Pyroptosis is perhaps the most dramatic, powerful, and pro-inflammatory response a cell can execute, and thus the signals that activate inflammasomes must be among the most dangerous in human biology. Despite their extraordinary importance, however, the identities of several of these danger signals, and how they activate their respective inflammasomes, have yet to be fully established.
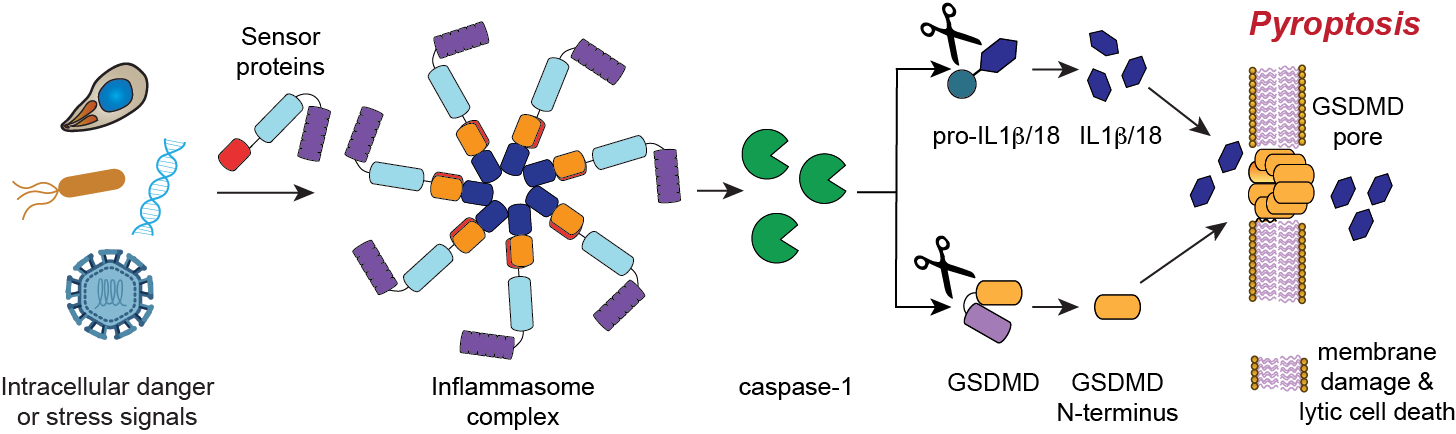
Fig. 1. Inflammasome activation. Certain pathogen-associated danger signals activate sensor proteins to form inflammasomes. Inflammasomes activate caspase-1, which in turn cleaves and activates pro-inflammatory cytokines and gasdermin D (GSDMD). GSDMD forms pores in the cell membrane, releasing the activated cytokines and inducing a lytic form of cell death called pyroptosis.
As a chemical biology lab, we entered the inflammasome field in an unusual way – via investigations into the intriguing immunostimulatory activity of the small molecule Val-boroPro (VbP). VbP is a non-selective inhibitor of the post-proline cleaving serine proteases that induces immune-mediated tumor regressions in multiple mouse models of cancer. Although VbP’s anti-cancer activity was first identified more than twenty years ago, its mechanism of action was essentially unknown. We anticipated that characterization of VbP’s mechanism of action would be highly significant because it would reveal new fundamental mechanisms regulating mammalian immune systems and identify new targets and strategies for cancer immunotherapy. Shortly after starting my lab, we discovered that VbP stimulates immune systems by activating the related NLRP1 and CARD8 inflammasomes. Prior to this discovery, very little was known about the NLRP1 and CARD8 inflammasomes, including, most importantly, the identities of the danger signals that they detect. Over the past eight years, we have made considerable progress in characterizing these two innate immune receptors, as well as in delineating mechanisms that control inflammasome signaling in general. My lab is now working to fully understand, and eventually to therapeutically harness, the activation of the NLRP1 and CARD8 inflammasomes.
Current Projects:
- NLRP1 and CARD8 inflammasomes
- DPP9 inhibitors
- Programmed cell death pathways
Bio
Dr. Bachovchin received his Ph.D. in 2011 from The Scripps Research Institute under the guidance of Dr. Benjamin F. Cravatt. In the Cravatt lab, he developed and applied activity-based protein profiling approaches for the discovery of selective enzyme inhibitors. From 2011-2015, Dr. Bachovchin was a postdoctoral fellow in Dr. Todd Golub’s laboratory at The Broad Institute. In the Golub lab, he developed high-throughput methods for profiling inhibitor selectivity and characterized compounds with enigmatic biological activities. At SKI, his lab uses chemical and cell biology approaches to study proteins and pathways involved in innate immunity, in particular inflammasomes.
Distinctions:
- David P. Hajjar Excellence in Teaching and Mentoring Award, Weill Cornell Graduate School, May 2023
- Geoffrey Beene Junior Faculty Chair, March 2022-March 2026
- Louise and Allston Boyer Young Investigator Award for Distinguished Achievement in Basic Research, June 2021
- Pershing Square Sohn Prize for Young Investigators in Cancer Research, July 2018-June 2021
- Alfred P. Sloan Foundation Fellow in Chemistry, February 2018
- Pew-Stewart Scholar Award for Cancer Research, July 2017-June 2021
- Stand Up to Cancer (SU2C) Innovative Research Grant, July 2017-June 2020
- Josie Robertson Investigator (MSKCC), September 2015 – August 2020
