
Research
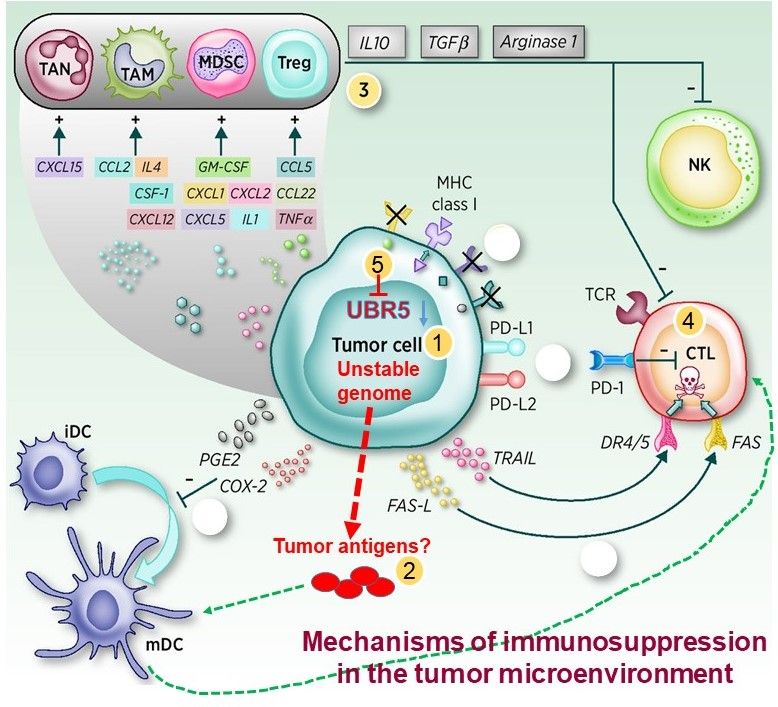
Traditional cancer therapy is organ- and tissue-based, which has been ineffective, and which also frequently results in therapy resistance and disease relapse. A new paradigm is emerging that combinations of key mutations in critical biological pathways may underlie broad arrays of malignant development and aggression. Targeting these common and vital points of tumorigenic regulation may yield greater impact on cancer therapy across the spectrum than aiming at narrowly focused, individual cancer-specific targets if systemic toxicity can be avoided. Our recent clinical and laboratory investigations have identified UBR5, a novel HECT domain E3 ubiquitin ligase of fundamental importance, as an essential cross-cancer regulator of tumorigenesis and anti-tumor immunity. UBR5 gene alterations occur in 25-35% of breast, ovarian and prostate cancers. In addition, genetic lesions in UBR5 have been found strongly associated with reduced survival in cancer patients. Yet, how UBR5 promotes cancer progression is highly obscure. We are using state-of-the-art technologies and experimental strategies to explore the pathophysiology and immunobiology of this enigmatic molecule of great clinical potentials. We are exploring UBR5’s immunological role and identifying “neoantigens” induced in UBR5-targeted tumors that may trigger T cell responses for prospective cancer vaccine development and immunotherapy. Targeting the UBR5 pathway will not only bring immediate clinical benefits to patients of a wide array of highly aggressive and therapy-resistant malignancies by abrogating cancer growth and metastasis but also lead to immune activation that will result in the generation of T cell memories and delicate immunosurveillance against cancer recurrence and drug resistance.
Figure 1
Current Projects:
- To identify UBR5’s E3 ligase substrates and interacting protein partners.
- To identify potential tumor-associated antigens induced in UBR5-deficient tumors that trigger T cell responses for future cancer vaccine development and immunotherapy.
- To investigate the immunoregulatory activities of UBR5 on myeloid cells in the tumor microenvironment.
- To explore the role of UBR5 in the development of T cell lineages and their function in tumor immunity.
- To identify molecular “degraders” of UBR5 via Proteolysis Targeting Chimera (PROTAC) for therapy.
Bio
Xiaojing Ma earned his Bachelor of Science degree in Animal Science from Beijing Agricultural University in China in 1982, and PhD in Molecular Genetics from University of Edinburgh, UK in 1987. From 1988 to 1999, he worked at the Wistar Institute in Philadelphia first as a Postdoc in Molecular Biology, then a Research Associate and Senior Scientist in the Immunology Program where he joined the effort to discover the cytokine interleukin-12 and explored its immunobiology. In 2000, Xiaojing Ma was recruited to Weill Cornell Medicine’s Department of Microbiology and Immunology as an Assistant Professor and rose through the ranks to become a tenured professor in 2007.
