
Research
Cancer is a complex interconnected ecosystem- a community in which tumor cells cooperate with other tumor cells and host cells in their microenvironment (TME) that contributes to tumor progression and metastasis. We are dissecting the complexity of the ecosystem, with the express goal to identify specific vulnerabilities that can be exploited for therapy development. We are dissecting the complexity at single cell resolution using genome-wide transcriptomics, proteomics, metabolomics, and epigenetics. Another layer of complexity includes intratumoral heterogeneity (ITH), where specific regions within the tumors show specialized microenvironments. Given that ITH is a major obstacle to effective cancer treatment, we are characterizing ITH using single cell spatial approaches (spatial transcriptomics, Hyperion imaging and MALDI Imaging mass spectrometry). Our research employs preclinical models, patient-derived organoids and human specimens from clinical trials.
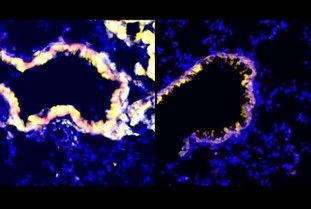
Figure 1 Immunofluorescent images of lungs treated with radiation (left) or no radiation (right). Clubs cells secrete more anti-immunisuppresive factors when treated with radiation, as seen by the increase in the secretory marker CC10 (yellow).
Metastasis is the major cause of mortality in triple-negative breast cancer (TNBC) patients. Despite the clinical significance, the identity of metastasis-initiating cells has remained intractable. Identification and characterization of metastatic compartment in TNBC has the potential to facilitate development of novel metastasis-specific therapies. Using the lineage tracing approach, we have identified within the primary tumor, a discrete population of cells with enhanced metastatic potential. We have shown that these cells have elevated expression of the master epigenetic modulator, the polycomb repressive complex 2 (PRC2) holoenzyme, and EZH2 inhibition with clinical grade drugs impaired metastatic colonization and outgrowth. These metastatic cells also exhibit increase chromosomal instability (CIN), a hallmark of cancer metastasis, and through a collaboration with the Bakhoum Lab (MSKCC), we recently showed that EZH2 epigenetically regulates CIN by inducing centrosome amplification and multipolar mitosis which often clustered into pseudo-bipolar mitotic spindles exhibiting chromosome segregation defects. We are exploring the potential of developing of first CIN suppressive treatment approaches for cancer.
We have also identified altered metabolic rewiring in the metastatic compartments particularly increased oxidative phosphorylation (OXPHOS) and fatty acid metabolism (synthesis and oxidation). Importantly, both these pathways are metal dependent. We already showed that copper (Cu) depletion impacts OXPHOS associated with metastasis inhibition in both mice and human. Currently, we are dissecting the Cu-metabolism-metastasis axis to determine its potential as a next-generation therapeutic approach for high-risk TNBC patients.
In the lung cancer space, we are focused on disabling immunosuppressive mechanisms to increase efficacy of immune checkpoint blockade. We have recently identified a novel mechanism, whereby secretory function of lung resident club cells overcome immunosuppressive myeloid suppressor cells to restore CD8 T cell cytotoxic function. Currently, we are hunting for the club cell factor/s that may be developed as selective and specific inhibitors of myeloid cells. In another project, we have identified the pentose phosphate pathway (PPP) as novel metabolic pathway that skews CD8+ T cells towards a TCF1high population and PPP agonism in combination with PD-1 blockade impaired tumor growth. We have identified increased activation of the IRE1a-XBP1 sensor of endoplasmic reticulum (ER) stress pathway in cancer cells blunts expression of protective type-I IFN to bolster immunosuppression in the TME. We are also investigating premalignant lung nodules and are generating mRNA LNP vaccines for immunoprevention.
Through close partnership between basic scientists and clinicians, we are generating a deeper molecular understanding of malignant disease. Our innovative research has led to key discoveries, which are contributing to the design of future clinical trials and providing targets for the development of novel therapies. These findings have the potential to impact treatments, improve quality of life and reduce mortality in cancer patients.
Current Projects:
- Metabolic alterations in immune and cancer cells.
- Endoplasmic reticulum stress and immunotherapy
- Epigenetic regulation of chromosomal instability.
- mRNA vaccines to intercept premalignant lung cancer.
- Metals and cancer. Developing a global cancer metal map.
Bio
Dr. Mittal received post-doctoral training at Cold Spring Harbor Laboratory, New York in 1999, and was soon promoted to Assistant Professor. In 2008, he was recruited as Associate Professor and Director of Research, Neuberger Berman lung cancer Center at WCM. Mittal is currently a tenured Professor in the Department of Cardiothoracic Surgery. Dr. Mittal’s discoveries have been published in leading journals including Nature, Science, Nature Cancer, Cancer Discovery and Cancer Cell.
Distinctions:
- University Grants Commission Fellowship
- Co-Chair, Session on Biology of Metastases, ASCO Breast cancer Symposium
- Plenary speaker, The Australian Health Medical Research Congress\
- Dr V Ramalingaswami INSA Chair Awardee
