
Research
Cardiac arrhythmias remain a leading cause of mortality and morbidity. Cardiac arrhythmogenesis is complex with arrhythmia susceptibility depending on a large range of factors, including age, sex, race, genetic mutations, hormonal modulation, electrolyte imbalance, and autonomic imbalance. Therefore, it can be challenging to predict an individual's risk of developing arrhythmias. Similarly, the diversity within a population can influence treatment outcomes for a specific patient, including the efficacy of antiarrhythmic medications.
As a means to develop a quantitative understanding of the impact of population heterogeneity on arrhythmogenesis, one of our focus areas is development of cell-specific mathematical models of cardiomyocytes. Our current approach includes combining automated parameter identification methods with complex data objectives. The modeling work is closely coupled to development of strategies for best recording electrical activity in cardiac myocytes, with experimental protocols optimized for model calibration and validation. We then use the models to provide new insights into physiological and pathological differences among individual cells.
Another line of research focuses on determining pro-arrhythmia risk of new drugs. Before advancing to clinical trials, drugs must undergo pre-clinical testing of pro-arrhythmic risk. New strategies and regulations for pre-clinical screening are under development to improve specificity and provide mechanistic insights into drug effects. Our work in this area has included optimizing mathematical models to clinical data from patients with and without a congenital pro-arrhythmic syndrome to more accurately detect arrhythmic risk of ion-channel blocking drugs. In a related project, we applied optimization methods to develop experimental protocols that allow identification of pro-arrhythmia risk and ionic current block, thus developing a candidate drug screening approach that simultaneously gives risk and mechanism of action for each drug.
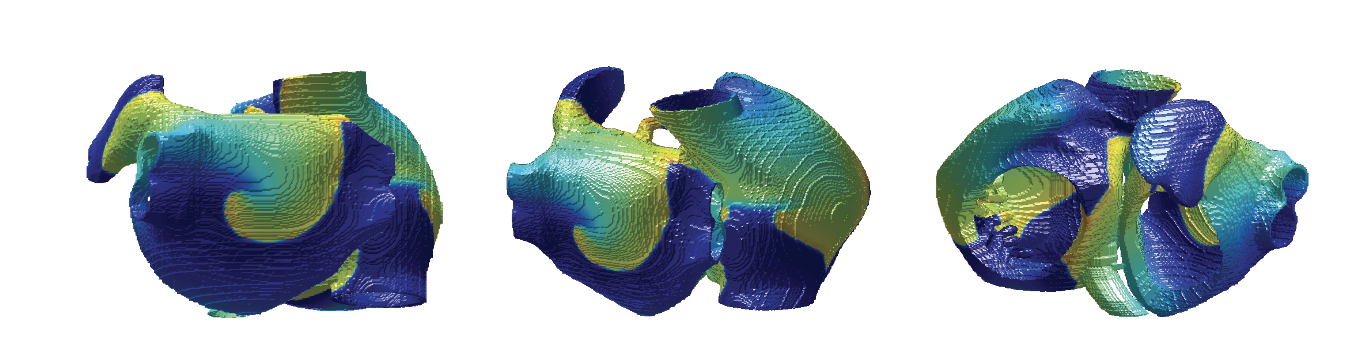
Figure 1: Simulation of arrhythmias in 3D anatomical model of the human atria
Current Projects:
- Cellular Digital Twins
- Inter-Cellular Heterogeneity
- Multi-Scale Cardiac Modeling
Bio
Krogh-Madsen completed her undergraduate studies and an MS degree in Applied Physics from the Technical University of Denmark in 1999. During this time, she developed an interest in mathematical modeling of biological systems and spurred by a semester spent at the Center for Nonlinear Dynamics in Physiology and Medicine at McGill University, enrolled in a physiology PhD program at McGill. After graduation (2004), Krogh-Madsen came to Weill Cornell as a postdoctoral fellow studying quantitative cardiac electrophysiology. She has been faculty at Weill Cornell since 2006.
Distinctions:
- Danish Research Academy Scholarship (2000)
- Member, National Institute of Mathematical and Biological Synthesis Working Group on Prediction and Control of Cardiac Alternans (2015-2017)
- Editor, Modeling and Simulating Cardiac Electrical Activity, IOP Publishing (2020)
Selected publications:
Krogh-Madsen T, Abbott GW, and Christini DJ. 2012. Effects of electrical and structural remodeling on atrial fibrillation maintenance: a simulation study. PLoS Computational Biology 8:e1002390.
Groenendaal W, Ortega FA, Kherlopian AR, Zygmunt AC, Krogh-Madsen T, and Christini DJ. Cell-specific cardiac electrophysiology models. PLoS Computational Biology, 11:e1004242.
Krogh-Madsen T, Sobie EA, Christini DJ. 2016. Improving cardiomyocyte model fidelity and utility via dynamic electrophysiology protocols and optimization algorithms. J Physiol. 594:2525-36.
Ip JE, Xu L, Dai J, Steegborn C, Jaffré F, Evans T, Cheung JW, Basson CT, Panaghie G, Krogh-Madsen T, Abbott GW, Lerman BB. 2021. Constitutively Activating GNAS Somatic Mutation in Right Ventricular Outflow Tract Tachycardia. Circ Arrhythm Electrophysiol. 14(10):e010082.
- Clark AP, Wei S, Kalola D, Krogh-Madsen T, and Christini DJ. 2022. An in silico–in vitro pipeline for drug cardiotoxicity screening identifies ionic pro-arrhythmia mechanisms. Br J Pharmacol. 197:4829.
