
Research
Our lab seeks to better understand the mechanistic basis of systemic lupus erythematosus (SLE), focusing on the relationship between skin disease and systemic autoimmunity. Two thirds of lupus patients develop inflammatory skin lesions with sun exposure and this skin sensitivity is associated with sun-induced flares of systemic disease. The skin communicates directly with the immune system by sending signals to draining lymph nodes, and study mechanisms of photosensitivity and the ways by which the state of the skin modulates draining lymph node (auto)immune responses. We study the interactions between immune cells and non-hematopoietic tissue stromal cells in skin, lymph nodes, and the lymphatic connections between them, with the goal of understanding how these complex interactions impact autoimmunity and inflammation in disease.
In the skin, we have shown that Langerhans cells, independent of their antigen-presentation functions, protect the skin by limiting ultraviolet radiation (UVR)-induced keratinocyte apoptosis via the metalloprotease ADAM17. ADAM17 clips and activates epidermal growth factor receptor ligands to promote keratinocyte survival, and Langerhans cells in lupus models show ADAM17 dysfunction, leading to photosensitivity (Shipman et al, Sci Trans Med 2018). In ongoing work, we are showing that type I IFN elevated in SLE causes Langerhans cell ADAM17 dysregulation and our work suggests that the recently FDA-approved anifrolumab (anti-IFNAR-1) may have beneficial effects on SLE skin in part by restoring ADAM17 activity.
To better understand how sun exposure can lead to increased autoantibody titers and systemic disease flares in SLE, we are now also studying the lymphatic vessels that function to clear skin of inflammatory mediators and bring antigens and other information to draining lymph nodes to shape immunity. We have found evidence of reduced lymphatic flow in human and multiple mouse models of SLE. Correcting lymphatic flow with manual lymphatic drainage (MLD) or in a transgenic model reduces photosensitivity and lymph node B cell responses, in part by acting on lymph node fibroblastic reticular cells (FRCs). We are currently studying how lymphatic flow modulates other aspects of lymph node function and are interested in understanding whether MLD could be used as a therapeutic approach in SLE.
Figure 1
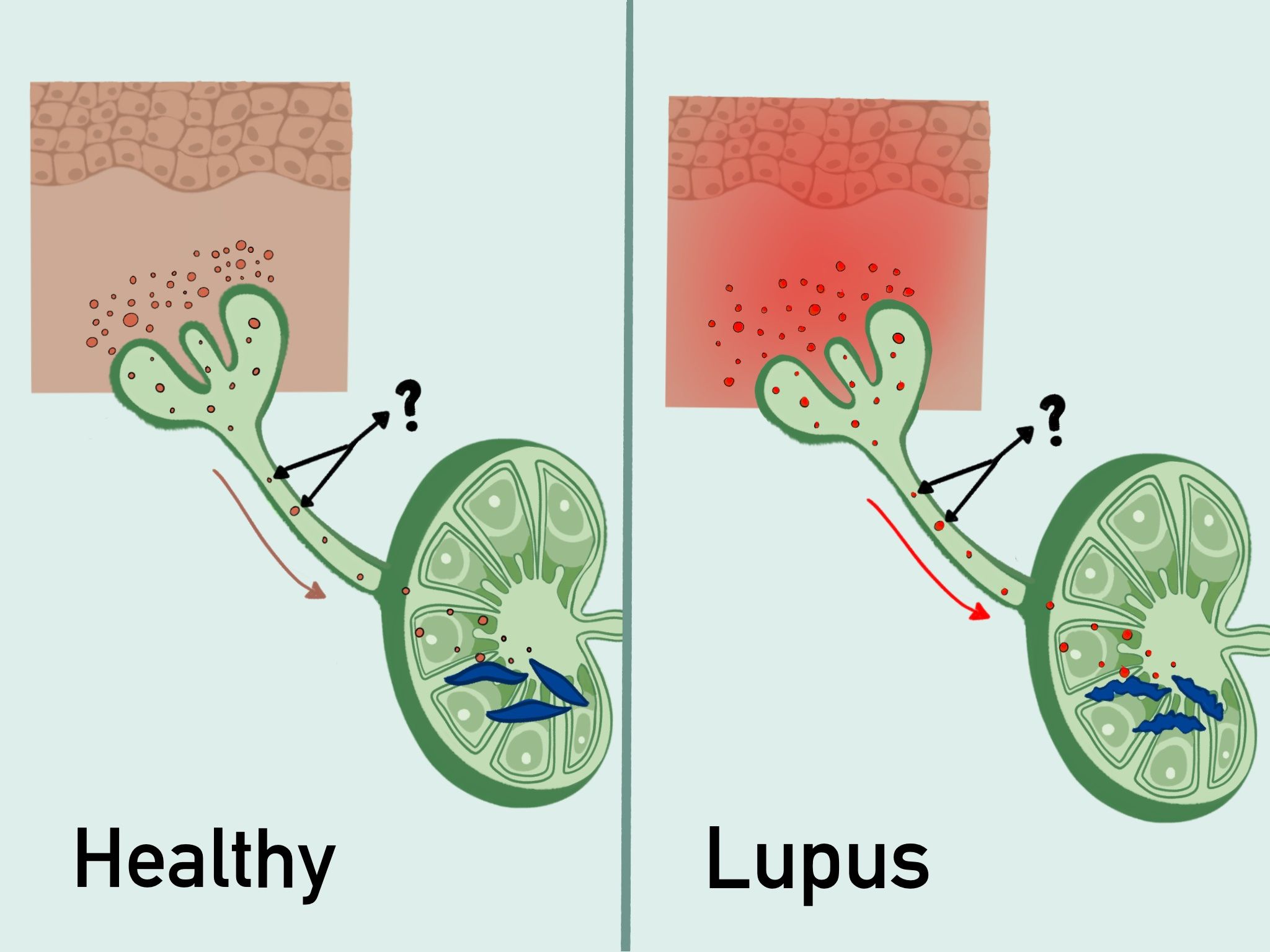
Current Projects:
- Langerhans cells
- Lymphatics
- Skin-lymph node axis
- Lymph node stromal cells
Bio
Theresa Lu holds the St. Giles Chair for Research in the HSS Research Institute and is Professor of Microbiology and Immunology and Professor Pediatrics at Weill Cornell Medicine. She is also currently Co-Director of the Weill Cornell PhD Program in Immunology and Microbial Pathogenesis. Dr. Lu received her MD and PhD from Yale University, trained in Pediatrics at Children's Hospital of Philadelphia, and completed her fellowship in Pediatric Rheumatology at the University of California San Francisco. She completed her PhD training with Joseph Madri at Yale and her postdoc training with Jason Cyster at UCSF. She has received numerous awards, including the American College of Rheumatology Young Investigator Award, Weill Cornell Teaching and Mentoring Award, and induction into the American Society for Clinical Investigation and the Henry Kunkel Society for immune disease research. Dr. Lu's goals are to better understand and treat lupus and other autoimmune diseases and support the growth and development of the next generation of scientists.
Distinctions:
- 2023 Inaugural speaker, Lucille and Joseph Madri Lecture, Yale University
- 2020 St. Giles Research Chair
- 2015 Henry Kunkel Society for immune disease research, membership
- 2014 Jean Davis Research Grant from the Alliance for Lupus Research
- 2011 Henry Kunkel Young Investigator Award, American College of Rheumatology
- 2011 American Society for Clinical Investigation, membership
- 2010 Weill Cornell Graduate School Dean’s Award for Teaching and Mentoring
