
Research
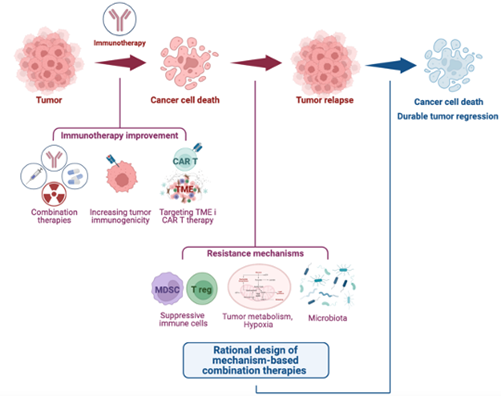
Cancer cells employ different strategies to evade the immune system, including activating inhibitory checkpoint molecules like PD-1 and CTLA-4 in cytotoxic T cells, or losing immunogenic antigens. Immune-based therapies, enhancing anti-tumor immunity, are frontline treatments for many cancers due to their striking clinical efficacy. However, relapse remains a challenge. How do tumors become resistant to immunotherapies? Our lab seeks to address this, focusing on melanoma as a model and translating our knowledge to other tumors, including lung, breast, and bladder cancers. Our previous research has uncovered prominent roles for myeloid-derived suppressor cells (PMID: 27828943), regulatory T (Tregs) cells, and tumor metabolism, specifically glycolysis (PMID: 33588426) and tryptophan catabolism (PMID: 32782249), in limiting the efficacy of immune checkpoint blockade. We are further exploring how metabolic pathways and microbiota-derived metabolites impact on immunotherapy response.
Other major interest is tumor antigenicity. Tumor cells evade immune recognition by losing antigens, becoming “immune escape variants” driving resistance. Our group has discovered that T cell co-stimulation through OX40 induces neutrophil infiltration, which eliminates “immune escape variants” (PMID: 37001503), prompting us to explore neutrophil mobilization strategies. We are also developing approaches to increase tumor antigenicity, like delivering tumor antigens via commensal bacteria, and predict how functional T cell repertoires can inform of immunotherapy responses and help design tumor vaccines. Additionally, we are monitoring how antigen stimulation induces differentiation of single-to-double positive T cells with polyfunctional characteristics (PMID: 35604411), which will aid identifying antigen-specific T cells and pathways underlying their functional plasticity and clinical relevance.
Advancing strategies for enhancing immunotherapies is a key goal. We are investigating the efficacy of combining T cell co-stimulation, chemotherapy, and targeted therapies with checkpoint blockade. Our previous work has revealed that cyclophosphamide with GITR-mediated T cell co-stimulation induces immunogenic tumor cell death, expanding oligoclonal, tumor-infiltrating cytotoxic T cells (PMID: 34676831). We are now examining cyclophosphamide and checkpoint blockade combo efficacy. We have also demonstrated that RAF/MEK inhibition with CTLA-4 blockade enhances T cell function in RAS pathway mutant tumors (PMID: 24416731; PMID: 30995478) and are currently evaluating mutant KRAS inhibitors with immunotherapy for lung cancers. Furthermore, we are engineering chimeric antigen receptor (CAR) T cells for melanoma, glioblastoma and prostate cancer and evaluating their therapeutic potential with combination strategies targeting the immunosuppressive tumor microenvironment. We hope our findings enable us to initiate clinical trials leveraging the potential of the immune system for effectively managing and eradicating solid tumors.
Current Projects:
- Myeloid derived suppressor cells and regulatory T cells in tumor immunity.
- Impact of tumor metabolism on immunotherapy response.
- Tumor antigenicity and approaches to predict antigen-specific, functional T cells.
- Optimal cancer vaccine strategies in pre-clinical mouse models.
- New strategies combining immunotherapy with chemotherapy and targeted agents.
- Leveraging T cell co-stimulation to improve immunotherapies.
- Targeting the tumor microenvironment to improve CAR T cell therapy.
Bio
Dr. Taha Merghoub is the Deputy Director of the Meyer Cancer Center at Weill Cornell Medicine (WCM), New York. He is also Professor of Pharmacology, Professor of Immunology Research in
Medicine and Margaret and Herman Sokol Professor of Oncology Research at Weill Cornell Medicine (WCM), New York. He is the director of the Ludwig collaborative and swim across America
laboratory at WCM. He received his BA degree from the University of Algiers, Algeria, and MS and PhD degrees with highest distinction from University of Paris, France. His thesis work focused on the study of genetic polymorphism in fetal hemoglobin gene in patients with sickle cell anemia and thalassemia. His findings provided insight to the correlation of genotypes and phenotypes in sickle cell anemia and thalassemia. After graduation, he pursued his postdoctoral research with Dr. Pier Paolo Pandolfi at Memorial Sloan Kettering Cancer Center (MSK). He characterized the transcriptional properties of the Pokemon (Zbtb7) gene and its role during development. He also played an active role in the generation of laboratory models for acute promyelocytic leukemia and furthered his knowledge and experience in genetics. He subsequently joined Alan Houghton laboratory as a Senior Research Scientist where he started studying tumor immunity. He developed mouse models of melanoma that mimic different stages of human disease clinically, pathologically and genetically, later evaluating new immunotherapies at different stages of tumor progression. He then became an assistant attending biologist in the melanoma and sarcoma service and assumed the direction of
a research laboratory in partnership with Dr. Wolchok. He successively became associate attending in the melanoma and immunotherapeutic service to then become a full member in the Human Oncology and Pathogenesis Program (HOPP) department at MSK. He was also a Parker Institute member researcher and led the Melanoma disease management team (DMT) and the Immunotherapeutic group tissue bank at MSK. He was appointed in his current role at WCM in September 2022. He directs a translational tumor immunology laboratory at WCM. His current research projects focus on investigating the means for developing immune-based therapies to treat cancer. In addition, some of his work aims to study the pathogenesis and treatment of melanoma and is directed in part at developing tools to study melanoma and immune responses in multiple cancers. His projects are heavily collaborative in nature, within the institution and across different disciplines with multiple entities. His projects can be divided into the following categories: 1) overcoming resistance to immune checkpoint blockade therapy by modulating the immune system; 2) using the immune modulatory properties of modalities that target and kill tumor cells directly; 3) defining biomarkers and genetic determinants of response to immune therapy; and 4) developing a tissue repository for the immunotherapy and melanoma groups. Dr. Merghoub's findings have been published in prestigious journals including Science, Nature, Cell, Nature Medicine, Nature Immunology, Cancer Cell. He has an h-index of 75, over 35 830 citations which is considered at the top2% worldwide.
Dr. Merghoub participated in multiple actions and initiatives to build bridges between the United
States and Algeria. Over the years, he contributed to multiple organization including the Algerian
American Foundation for Culture, Education, Science and Technology (AAF-CEST). He served on
the board of the AAF-CEST from 2014 to 2021 and as AAF-CEST president from 2017 to 2020.
Distinctions:
Selected Publications:
1. Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schröder D, Chow A, Ricca JM, Gasmi B, De Henau O, Mangarin LMB, Li Y, Hamadene L, Flamar AL, Choi H, Cortez CA, Liu C, Holland A, Schad S, Schulze I, Betof Warner A, Hollmann TJ, Arora A, Panageas KS, Rizzuto GA, Duhen R, Weinberg AD, Spencer CN, Ng D, He XY, Albrengues J, Redmond D, Egeblad M, Wolchok JD, Merghoub T. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. 2023 Mar 30;186(7):1432-1447.e17. doi: 10.1016/j.cell.2023.03.007. PMID: 37001503.
https://pubmed.ncbi.nlm.nih.gov/37001503/
2. Hoyos D, Zappasodi R, Schulze I, Sethna Z, de Andrade KC, Bajorin DF, Bandlamudi C, Callahan MK, Funt SA, Hadrup SR, Holm JS, Rosenberg JE, Shah SP, Vázquez-García I, Weigelt B, Wu M, Zamarin D, Campitelli LF, Osborne EJ, Klinger M, Robins HS, Khincha PP, Savage SA, Balachandran VP, Wolchok JD, Hellmann MD, Merghoub T, Levine AJ, Łuksza M, Greenbaum BD. Fundamental immune-oncogenicity trade-offs define driver mutation fitness. Nature. 2022 Jun;606(7912):172-179. doi: 10.1038/s41586-022-04696-z. Epub 2022 May 11. Erratum in: Nature. 2022 May 31;: PMID: 35545680; PMCID: PMC9159948.
https://pubmed.ncbi.nlm.nih.gov/35545680/
3. Chow A, Schad S, Green MD, Hellmann MD, Allaj V, Ceglia N, Zago G, Shah NS, Sharma SK, Mattar M, Chan J, Rizvi H, Zhong H, Liu C, Bykov Y, Zamarin D, Shi H, Budhu S, Wohlhieter C, Uddin F, Gupta A, Khodos I, Waninger JJ, Qin A, Markowitz GJ, Mittal V, Balachandran V, Durham JN, Le DT, Zou W, Shah SP, McPherson A, Panageas K, Lewis JS, Perry JSA, de Stanchina E, Sen T, Poirier JT, Wolchok JD, Rudin CM, Merghoub T. Tim-4+ cavity-resident macrophages impair anti-tumor CD8+ T cell immunity. Cancer Cell. 2021 Jul 12;39(7):973-988.e9. doi: 10.1016/j.ccell.2021.05.006. Epub 2021 Jun 10. PMID: 34115989; PMCID: PMC9115604.
https://pubmed.ncbi.nlm.nih.gov/34115989/
4. Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, Watson MJ, Leftin A, Maniyar R, Verma S, Lubin M, Ko M, Mane MM, Zhong H, Liu C, Ghosh A, Abu-Akeel M, Ackerstaff E, Koutcher JA, Ho PC, Delgoffe GM, Blasberg R, Wolchok JD, Merghoub T. CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours. Nature. 2021 Mar;591(7851):652-658. doi: 10.1038/s41586-021-03326-4. Epub 2021 Feb 15. PMID: 33588426; PMCID: PMC8057670.
https://pubmed.ncbi.nlm.nih.gov/33588426/
5. De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, Douglas M, Tibbitts T, Sharma S, Proctor J, Kosmider N, White K, Stern H, Soglia J, Adams J, Palombella VJ, McGovern K, Kutok JL, Wolchok JD, Merghoub T. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016 Nov 17;539(7629):443-447. doi: 10.1038/nature20554. Epub 2016 Nov 9. PMID: 27828943; PMCID: PMC5634331.
