
Research
The Jaffrey laboratory is interested in how regulation of mRNA stability and translation impacts gene expression in normal tissues and disease. Deregulation of RNA-regulatory pathways has been shown by us and others to lead to neurological disfunction, cancer, autoimmune diseases, and other conditions. We have found that a major RNA regulatory mechanism is the chemical modification of mRNA to form modified nucleotides. These modified nucleotides are recognized by specific proteins to affect the stability or translation of these mRNAs in cells. We termed this field “epitranscriptomics,” i.e., the study of the role and function of mRNA base modifications. Our transcriptome-wide map of N6-methyladenosine (m6A) was the first global analysis of an mRNA base methylation and helped to initiate widespread interest in m6A as a regulatory mechanism for mRNA and ncRNAs. We found that, m6A, a widespread modification that occurs in over 7000 transcripts in cells. We showed that the major function of m6A in mRNAs is to mediate mRNA degradation, but can also regulate mRNA translation in specific circumstances. We showed that m6A can markedly enhance the ability of noncoding RNA to exhibit epigenetic effects on gene expression.
We also mapped other modified nucleotides throughout the transcriptome including, m6Am and Cap2, a 2’-O-methyl modification on ribose sugars at the beginning of certain mRNAs. We are determining how m6A, Cap2 and m6Am control mRNA and protein expression in diverse cellular and disease contexts.
The Jaffrey lab is also interested in identifying RNA regulatory pathways that control protein expression in neurodevelopmental disorders such as mental retardation and autism. We showed that the cause of the abnormal gene silencing that causes the neurodevelopmental disorder Fragile X syndrome involves a novel pathway in which trinucleotide repeat RNA hybridizes to complementary DNA sequences. These studies identified a novel mechanism of epigenetic regulation that may be broadly relevant to repeat expansion disorders. We are interested in determining how RNAs in these and other disorders can have a toxic function that leads to cytotoxicity.
The Jaffrey lab also develops RNA aptamer technologies for probing and manipulating cellular biology. We developed a series of RNA aptamers that bind and activate the fluorescence of otherwise nonfluorescent small molecules. These RNA aptamers, most notably Spinach, function as RNA mimics of green fluorescent protein. We have created newer RNA-fluorophore complexes with novel spectral properties, such as Broccoli, Corn, and Squash, for studying RNA trafficking in living cells. We have also converted these aptamers into genetically encoded biosensors that fluoresce upon binding intracellular metabolites. We also develop novel RNA synthetic biology tools, including the Pepper aptamer, which directly regulates the stability of target proteins, and the Tornado RNA circularization technique, which allows RNA aptamers to be expressed as highly stable circles. The high expression of these RNA aptamers allow them to control protein function. Our major goal is to develop new RNA technologies to control and manipulate cellular function. Many of these technologies have potential as new types of RNA-based therapeutics.
Figure 1
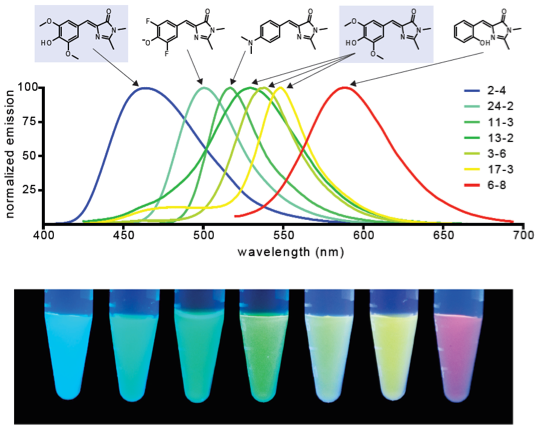
Figure 2
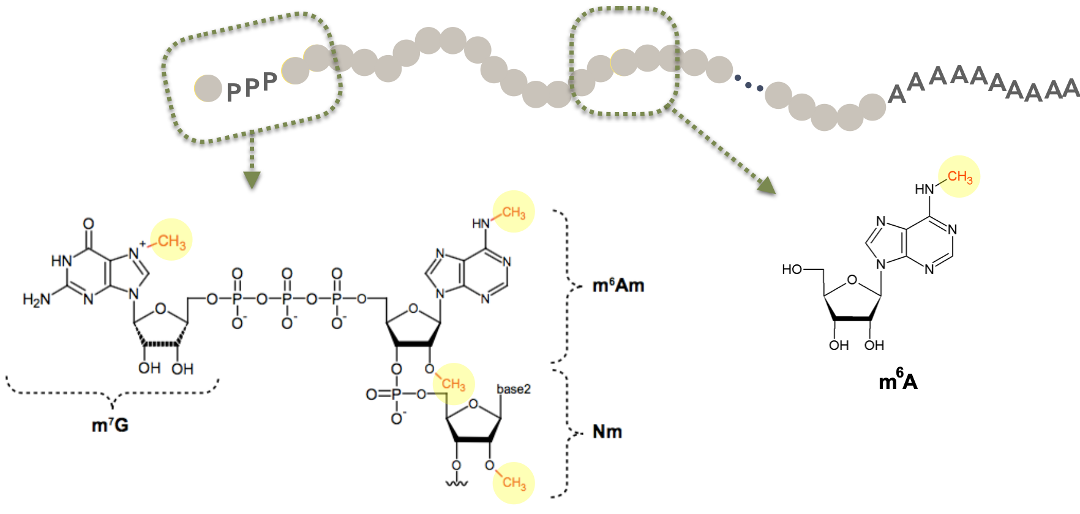
Current Projects:
- Epitranscriptomics
- mRNA modifications
- Post-transcriptional regulation
- RNA aptamers
- RNA therapeutics
- Circular RNA
Bio
Dr. Samie Jaffrey received his bachelor’s degree from MIT in 1992 and his MD and PhD from Johns Hopkins University in 1999 in the laboratory of Dr. Solomon Snyder. He joined the Department of Pharmacology at Weill Cornell Medicine in 2001. Dr. Jaffrey was named Greenberg-Starr professor in 2016.
Distinctions:
- John J. Abel Award in Pharmacology, American Society of Pharmacology and Experimental Therapeutics
- Elected Member, American Society of Clinical Investigation
- American Society for Biochemistry and Molecular Biology Young Investigator Award
- Blavatnik Award for Young Scientists
- NIH Director’s Transformative R01 award
- NIH EUREKA Award
- McKnight Foundation Technology Development Award
- Klingenstein Fellow in Neuroscience
