
Research
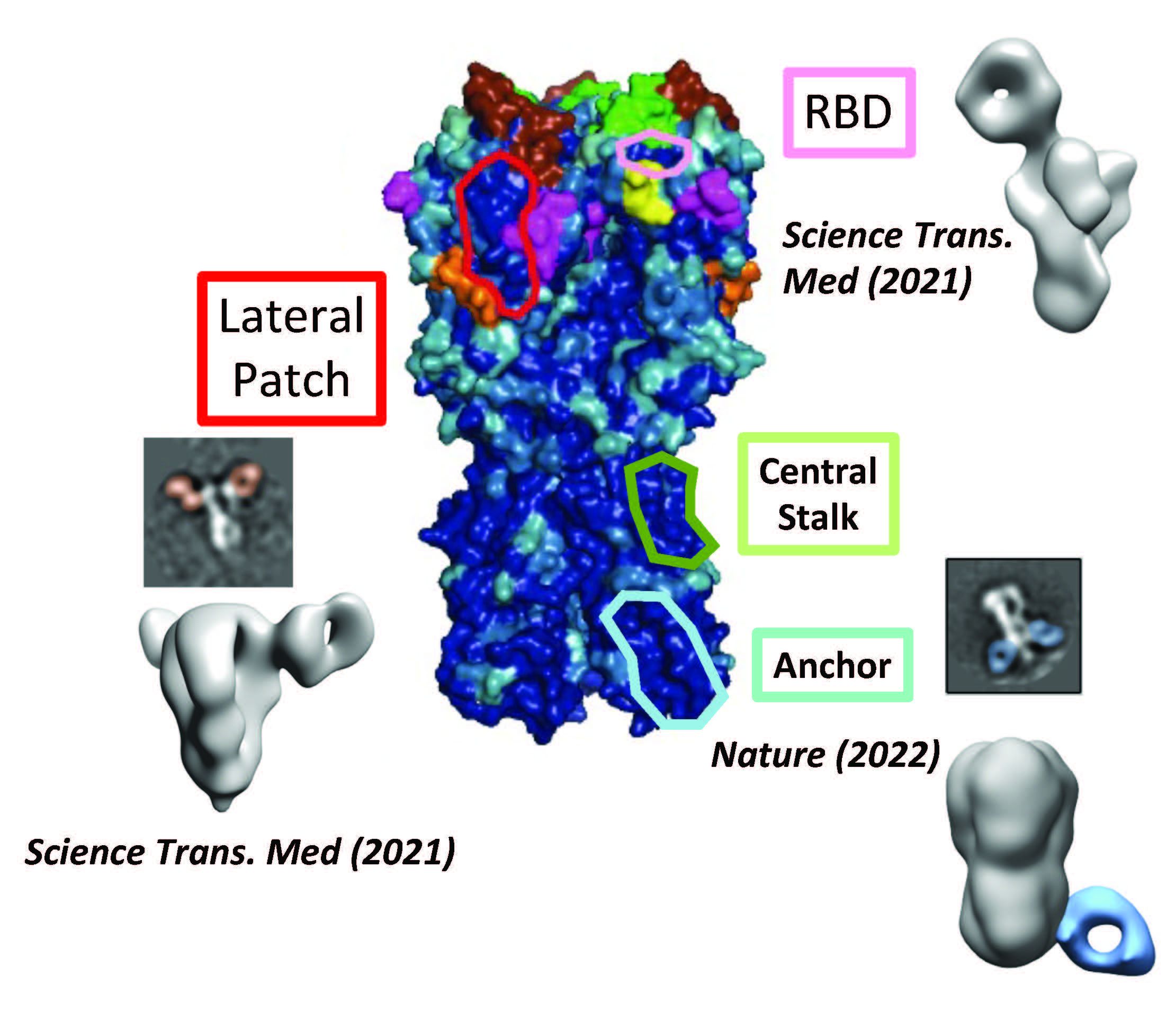
We apply various technologies and approaches to the study of autoimmunity, B cell biology, and responses to infectious diseases in humans and mice with a particular emphasis on influenza vaccinology. The genomes of influenza and now SARS-CoV-2 virus rapidly mutate, evolving new viral variants that escape from existing immunity from past exposures or vaccinations. Our immune responses are also biased by immune memory to target these evolved strains with antibodies from past immune responses that do not protect against the current viral strains. Together this “antigenic drift” and immune memory bias results in annual epidemics causing millions of infections and hundreds of thousands of deaths each year across the globe. The existence of large pools of even more distantly related influenza and coronavirus strains in animals represent the even greater threat of another pandemic as occurred in 2019, causing an estimated >16 million deaths. The 1918 influenza pandemic is estimated to have caused between 50-100 million deaths. A major interest of the Wilson lab is to understand viral immunity and to apply this knowledge to improved vaccines or antibody-based therapeutics that can protect us from all viral variants. A major component of this effort is the identification of novel antibodies targeting conserved epitopes that are resistant to viral mutation and so can protect against all viral strains. For example, and as illustrated, we have isolated many such antibodies to the influenza hemagglutinin protein used by the virus to attach to cells, including against the receptor binding domain (RBD), the lateral patch, the central stalk, and to a novel epitope we discovered in 2022, the anchor (Nature, 2022). We also study the immune contexts that can lead to better immunity to influenza such as by realizing that a major component of influenza immunity that was not being targeted by current vaccines is the neuraminidase protein (Cell, 2018). We continue to study influenza and COVID-19 immunity in humans and mouse models and to identify novel epitopes. Further, we have a vaccine development program for influenza. Finally, we have a major interest in pediatric immunity and autoimmune diseases. The Wilson laboratory typically approaches scientific questions about B cells from the standpoint of “specificity-first” through characterizing B cell and monoclonal antibody specificities for the interpretation of immune responses or to evaluate B cell tolerance. Our recent work has involved extensive use of single cell transcriptomics combined with cell surface and antigen binding applications. We then follow up with a multitude of approaches and technologies to characterize the B cells and antibodies involved in the responses or to evaluate novel vaccines.
Current Projects:
- B cells, antibodies, influenza, SARS-CoV-2, Immune Tolerance.
Bio
Dr. Wilson came into science in an atypical fashion, having put himself through college mostly at night school at Youngstown State University (1992) while working at a factory by day and serving in the Army Reserves to pay tuition. A lab tech job with Biology chair Tony Sobota at YSU while earning his MS (1994) led him to his career path. He then went on to Graduate School at the University Texas Southwestern Medical School with mentors Virginia Pascual and J. Donald Capra (1999). Dr. Wilson did his post-doctoral training in Michel Nussenzweig’s lab at the Rockefeller University.
Distinctions:
The Wilson laboratory has had a long-standing interest in understanding immunoglobulin repertoire diversity including important advances on how somatic mutation is targeted by antibody gene sequence elements, and as a graduate student under the mentorship of Virginia Pascual and Donald Capra, discovering that somatic hypermutation introduces insertions and deletions as well point mutations to antibody genes.
As a post-doctoral fellow in Michel Nussenzweig’s laboratory at Rockefeller and then in his own laboratory (i.e., Nature, 2008), Dr. Wilson and colleagues devised novel strategies for the cloning and expression of recombinant monoclonal antibodies. These technologies have led to major advances in hundreds of studies on understanding, vaccinating, and treating infectious diseases such as influenza, HIV, and COVID-19 and for understanding autoimmune diseases and immune tolerance mechanisms. The Wilson laboratory has now generated a repository of thousands of antibodies to influenza and SARS-CoV-2 that we have used or shared for dozens of publications advancing our understanding of immunity to these diseases.
In a first example published in the J. Exp. Medicine in 2011 and then in a series of papers thereafter, Dr. Wilson’s laboratory found that immunization with highly distinct influenza antigens such as avian influenza strains preferentially activate immune responses to highly conserved (universal) and protective epitopes. This observation is critical to show that targeted vaccine compositions can “retrain” and improve immunity to influenza, guiding efforts and showing feasibility for a “one-shot” universal influenza vaccine in the future.
In a Cell paper published in 2018, the Wilson laboratory reported that influenza vaccines rarely and ineffectively targeted the neuraminidase protein. However, we showed that antibodies to neuraminidase are highly protective to a wide range of influenza strains via mechanisms like drugs such as Tamiflu. Thus, a neuraminidase-based vaccine could provide a similar pre-existing reduction in disease severity. After this paper there has been a major effort to study neuraminidase immunity and to add a neuraminidase component to existing influenza vaccines including several ongoing or impending clinical trials.
Dr. Wilson was selected for a Bill and Melinda Gates Foundation Grand Challenge award to generate a novel universal influenza vaccine concept (2019).
In a 2022 Nature paper, we described the “anchor epitope” that is a highly conserved and protective epitope on influenza hemagglutinin that is now one of four major classes of antibody targets for potential universal influenza vaccine development or therapeutics.
