
Research
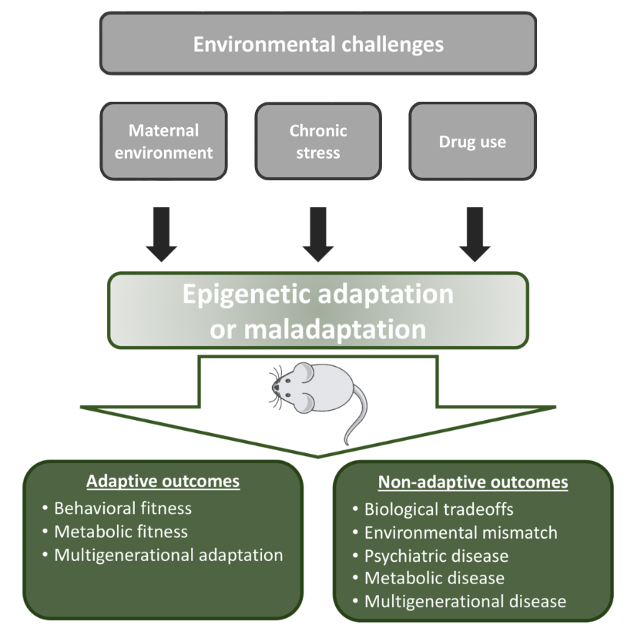
Humans encounter challenges in their environment through lifetime, including adversity in fetal life and childhood, and societal stress, psychological trauma, and exposure to drugs in adolescence and adult life. We identified thousands of small epigenomic regions in neurons that register environmental challenges and respond to them by altering their gene regulatory activity. This, in turn, produces adaptive or maladaptive transcriptional, neuronal, and behavioral responses. Adaptive responses facilitate coping with environmental challenges, while maladaptation leads to neuropsychiatric diseases such as depression, anxiety, and drug seeking/abuse behavior. We use a wide range of techniques in mouse models to mechanistically link environmental challenges, through epigenetic and transcriptional plasticity, to behavioral responses. These techniques include high throughput DNA methylation and chromatin profiling, single cell and bulk RNA expression, neuronal and synaptic physiology, and various behavioral assays. The ultimate goal is to understand epigenetic mechanisms that underly sensing, registering, and responding to environmental challenges and to identify pharmacological targets that enhances adaptive and alleviate maladaptive behaviors.
Figure 1
Current Projects:
- Environmentally sensitive transcriptional enhancers
- Registering environmental clues in the epigenome
- Epigenomic and neuronal states of adaptive and maladaptive behavior
Bio
Toth has an MD, PhD degree from Hungary and was a Humboldt Fellow in the University of Colone Germany and Howard Hughes fellow in Princeton University before joining the Department of Pharmacology, Weill Cornell Medicine, as Assistant Professor in 1993. He is a member of both the Pharmacology and Neuroscience Programs of WCGS.
Distinctions:
- 1988-1990 Alexander von Humboldt Fellow (Germany)
- 1990-1992 Associate of the Howard Hughes Medical Institute
- 1996 and 2003 NARSAD Investigator
- 2003-and 2004 FRAXA Foundation Investigator
- 2009 and 2013 EUREKA (Exceptional, Unconventional Research Enabling Knowledge Acceleration) grant recipient
