
Research
Dr. Gudas and her lab members have s a long-standing research interest in vitamin A (retinol) and its derivatives and metabolites, compounds called retinoids. She is also fascinated by stem cells in the adult and their roles in both driving cancer and in cell repair/regeneration after tissue damage from alcohol, obesity, and carcinogen exposure. Her lab utilizes both cell culture and several different mouse models of cancer, metabolic disease, and kidney disease. A major goal of her lab is to develop new therapies to prevent and/or treat head and neck cancer, kidney cancer, and liver steatosis (fatty liver disease).
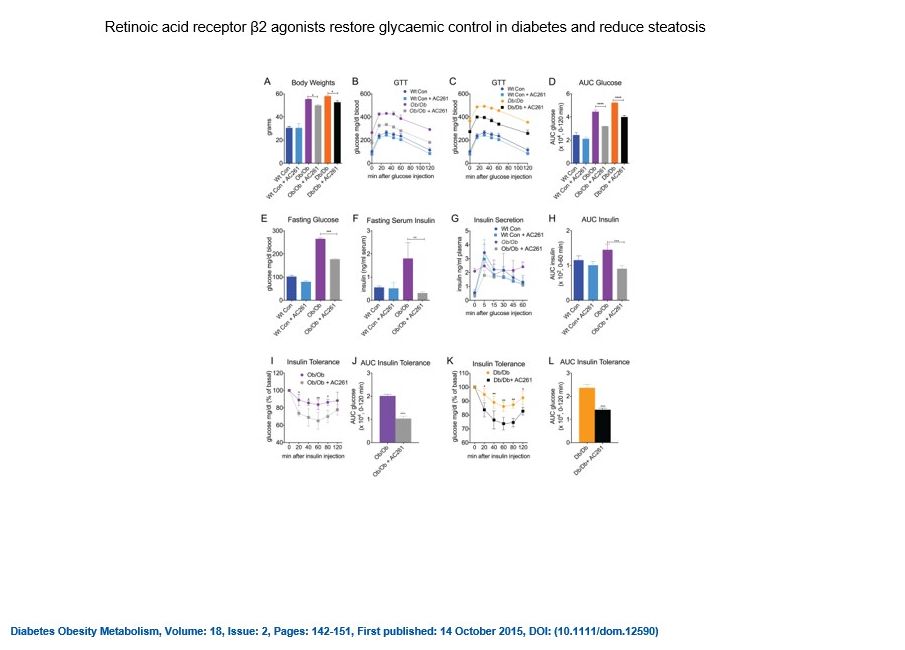
Current Projects:
- Analysis of the roles of stem cells in driving head and neck squamous cell carcinomas.
- Understanding the role of the transcription factor ATF4 in clear cell renal cell carcinoma, the most common form of kidney cancer.
- Delineating the actions of the mTOR pathway and Myc proteins in driving liver steatosis upon alcohol treatment.
- Deciphering epigenetic changes during stem cell differentiation.
Bio
Dr. Lorraine J Gudas PhD is the Chair and Revlon Pharmaceutical Professor of Pharmacology and Toxicology of the Pharmacology Department at Weill Cornell Medical College of Cornell University, New York City. She is a member of the American Society for Pharmacology and Experimental Therapeutics (ASPET), and she has served a term as an elected member of the Board of Directors of the American Association of Cancer Research, the largest organization of cancer researchers in the United States. Dr. Gudas also served as Chair of the Board of Scientific Counselors of NHLBI, and later as Chair of the Board of Scientific Counselors of the National Institute of Diabetes and Digestive and Kidney Disorders. She is a member of the external scientific advisory board of the Lineberger Cancer Center of U.N.C. Chapel Hill, and is also a member of the Scientific Advisory Board of the Waxman Cancer Research Foundation. Of note, in 1999, she received the 2nd Annual “Women in Cancer Research” award from the American Association of Cancer Research. Dr. Gudas was elected to be a Fellow of the AAAS in 2008. She served a term on the FDA Nonprescription Drugs Advisory Committee. She is also on the Editorial Board of Annual Review of Pharmacology and Toxicology. In 2020 she was elected to be a Fellow of ASPET. She is an Associate Editor of Cancer Research Communications.
