
Research
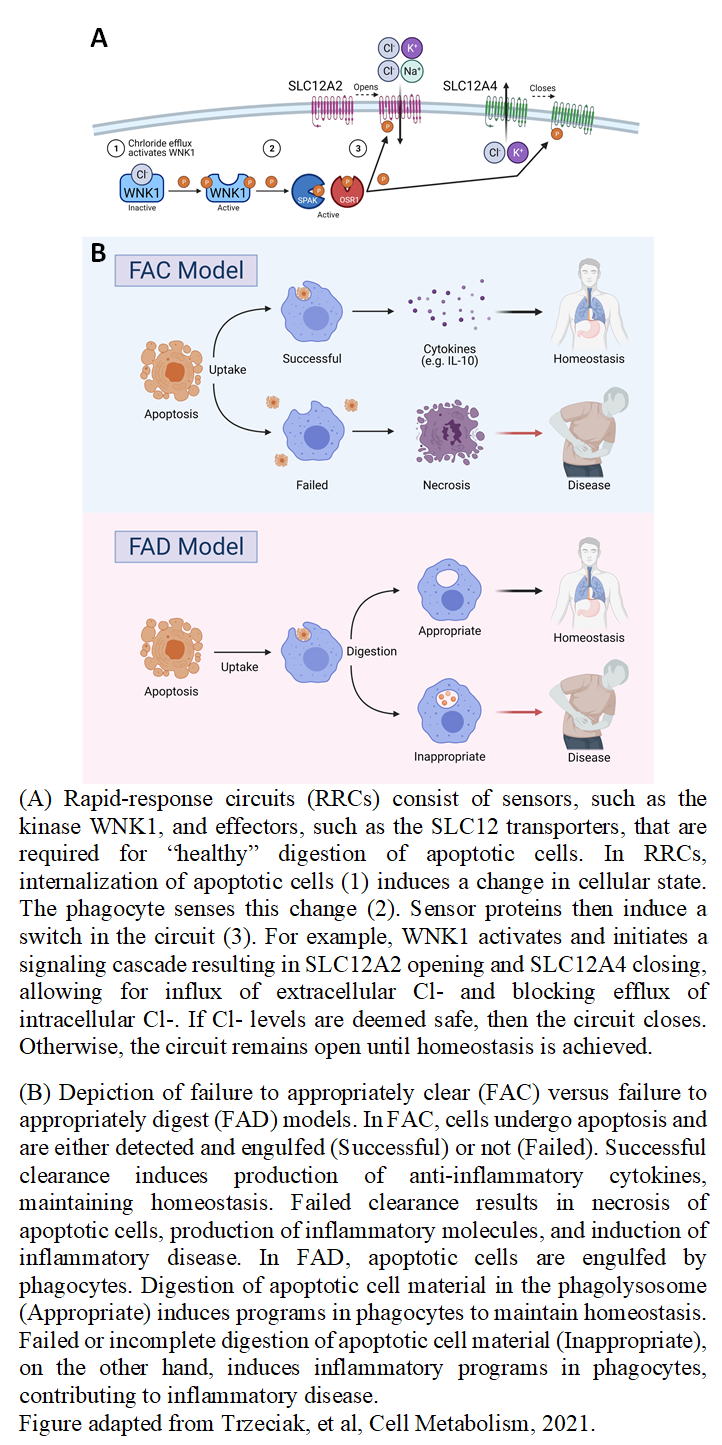
The human body is estimated to remove 1% of its body mass, likely more than 200 billion cells, every day. To achieve this, we rely on a highly evolutionarily conserved process: dead cell clearance or ‘efferocytosis’. Efferocytosis is essential for normal development and tissue homeostasis, but also pathogen defense and anti-tumor immunity. A single phagocyte often ingests an entire apoptotic corpse, essentially doubling its content. Because phagocytes often ingest multiple targets in succession, we propose that there are ‘rapid-response circuits’ composed of kinases that sense and activate in response to the change in a given solute, and solute transporters downstream of these kinases that impart the necessary flux of solutes (Figure 1A). We hypothesize that these circuits allow phagocytes to manage the immense risk dead cell and debris content poses to the homeostasis of both the phagocyte and ultimately the host (Figure 1B). The central scientific focus of the lab is to understand rapid-response circuits governing how phagocytes manage such excessive cargo influx. We aim to discover and understand novel phagocyte rapid-response circuits, to understand how the phagocyte’s environment regulates these circuits, how these circuits are critical for organismal development and maintenance of homeostasis across the lifespan, and how they falter or are exploited in diseases such as autoimmunity, inflammatory disease, and neurodegeneration. The Perry Lab uses an approach that starts from the broad and then focuses towards the specific, relying on combining informatics/biostatistics together with novel experimentation, new methods creation, and cutting-edge -omics approaches to identify and generate novel hypotheses about efferocytosis. We have developed extensive tools and reagents for in vitro testing of efferocytosis-associated targets. The lab’s core strength is our ability to perform cell biological, metabolic, and immunological studies of the programs induced during efferocytosis.
Figure 1.
Current Projects:
- How does tissue-specific macrophage metabolism inform phagocytosis?
- How do phagocytes manage the degradation of engulfed material?
- How do phagocytic and endocytic processes inform myeloid lineage commitment and maintenance?
Bio
Justin Perry, Ph.D., M.A., is an Assistant Member in the Immunology Program of the Sloan Kettering Institute (SKI) at Memorial Sloan Kettering Cancer Center and an Assistant Professor in the Immunology and Microbial Pathogenesis program at Weill Cornell Medical Center. Justin first obtained a M.A. in Clinical Psychology, followed by a Ph.D. in Immunology from Washington University in St. Louis. He then went on to complete a Postdoctoral Fellowship with Dr. Kodi Ravichandran at the University of Virginia.
Distinctions:
- NIH Office of the Director – New Innovator Award (DP2)
- Pew Charitable Trusts – Pew Biomedical Scholar
- V Foundation for Cancer Research – V Scholars Award
- Parker Institute for Cancer Immunotherapy – Career Development Award
- NIH/NCI K99/R00 Pathway to Independent Award
- Burroughs Wellcome Fund - Postdoctoral Enrichment Program Award Recipient
- Cancer Research Institute - Mark Foundation Postdoctoral Fellow
- Hanna Gray Fellow Finalist, Howard Hughes Medical Institute
