
Research
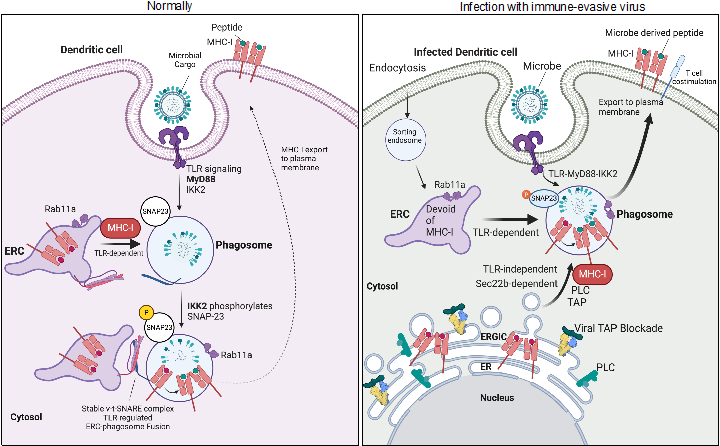
Regulation of Antigen Presentation: We were the first to demonstrate TLR control phagosome maturation kinetics, facilitating the delivery of internalized antigens to lysosomes for degradation and subsequent presentation by major histocompatibility complex (MHC) molecules. Our research on cross-presentation by DCs, uncovered unknown intracellular stores of MHC class I and defining the signals controlling their selective recruitment to phagosomes carrying TLR ligands. These findings led to the discovery of noncanonical cross-presentation as a cell autonomous pathway, allowing DCs to overcome viral immune evasion of MHC-I presentation and rescue CD8 T cell priming. Currently, we are leveraging this pathway in designing pancoronavirus vaccines.
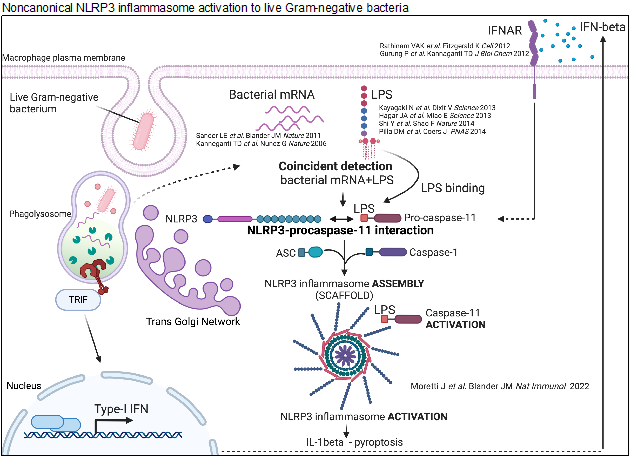
Vita-PAMPs in Vaccine Design: Our pioneering work introduced the concept of vita-PAMPs, molecular signatures exclusive to live microorganisms. Identifying bacterial mRNAs and cyclic-di-AMP as vita-PAMPs, we unveiled their role in triggering heightened innate and cellular stress responses in macrophages. Our research explained why live attenuated vaccines outperform their inactivated counterparts. Bacterial mRNA enhances dead vaccine efficacy by promoting follicular T helper cell differentiation and a robust B cell response. Vita-PAMPs hold potential to enhance current vaccines and design novel, safe adjuvants mirroring live vaccine efficacy.
Immune Consequences of Cell Death: We established that host cell apoptosis during infection influences the T helper 17 (TH17) response, linking apoptotic cell antigens to autoimmunity. Our findings challenged the conventional view of passive shedding of dying intestinal epithelial cells, revealing their active sampling by resident phagocytes. These insights into inflammatory pathways and immune suppression mechanisms have implications for understanding and treating Inflammatory Bowel Disease (IBD), emphasizing the importance of mucosal healing.
Innate Immune Barriers Against Malignant Cellular Transformation. Exploring innate immune defenses against early cancer development is a crucial but understudied area. Existing knowledge, mostly derived from established tumors in mice and humans, emphasizes inflammation's tumor-supportive roles and suppressed anti-tumor immunity. The lack of focus on early cancer stages is attributed to limited animal models replicating the transition from premalignancy to malignancy. Human data on immune regulation during pre-malignancy is scarce as patients typically seek medical attention when malignancy is established. Our lab is pioneering a new research direction by creating mouse models to investigate innate immune barriers in the premalignant phase.
Innate Immunity for Cancer Immunotherapy: In cancer immunotherapy, our lab devised a tumor cell vaccination strategy leveraging bacterial flagellin to simultaneously activate TLR and NLR pathways. This dual targeting induced potent anti-tumor CD4 and CD8 T cell responses, protecting mice against tumor challenges. In ongoing work, we identified novel inflammasome agonists that elicit potent adjuvant and anti-tumor activity, and are optimizing delivery methods for preclinical cancer vaccines and tumor microenvironment modulation.
Current Projects:
- The regulation of antigen cross-presentation by innate sensors
- Innate sensing of vita-PAMPs, signatures of microbial life
- Innate sensing and immune consequences of cell death
- Innate immunosurveillance against cancer
Bio
Blander is an expert in innate immunity and inflammation. Blander received her Ph.D. from the University of Pittsburgh studying tumor immunology under the mentorship of Olivera Finn. She conducted her postdoctoral training at Yale University with Ruslan Medzhitov and the late Charles Janeway, pioneering the study of the impact of Toll-like receptors on macrophage and dendritic cell function. The Blander lab was established in 2006 upon Blander’s appointment as Assistant Professor at the Mount Sinai School of Medicine. Blander became an Associate Professor with tenure in 2011, and an endowed full professor with tenure at Weill Cornell/Cornell University in 2016.
Distinctions:
- 2022 Outstanding Scientific Achievements Award, European Macrophage and Dendritic Cell Society
- 2021 Jeanne and Herbert Siegel Award for Outstanding Medical Research
- 2021 XSeed Award, Deerfield, Healthcare Investment Management Firm
- 2019 Sanofi iAward for Innovation, Sanofi
- 2017 Daedalus Award for Innovation, Weill Cornell Medicine
- 2011 Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award
- 2011 Mount Sinai School of Medicine Faculty Council Junior Faculty Award for Academic Excellence
- 2010 Harold and Golden Lamport Award
- 2010 Irma T. Hirschl and Monique Weill-Caulier Scholar Award
- 2009 Society of Leukocyte Biology: G. Jeanette Thorbecke Award
- 2007 Searle Scholar
Selected Publications:
Blander, J.M., Yee Mon, K.J., Jha, A., and Roycroft, D. (2023). The show and tell of cross-presentation Advances in Immunology [Epub 2023 Oct 12].
Barbet, G.*, Nair-Gupta, P.*, Schotsaert, M., Yeung, S.T., Moretti, J., Seyffer, F., Metreveli, G., Gardner, T., Choi, A., Tortorella, D., Tampé, R., Khanna, K.M., García-Sastre, A., and Blander, J.M. (2021). TAP dysfunction in dendritic cells enables non-canonical cross-presentation for CD8 T cell priming Nature Immunology [Epub 31 March 2021] *Equal contribution
Moretti, J., Jia, B, Hutchins, Z., Roy, S., Yip, H., Wu, J., Shan, M., Jaffrey, S., Coers, J., Blander, J.M. (2022). Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome Nature Immunology 23:705-717 [Epub 2022 April 29].
Blander, J.M. (2018) On cell death within the intestinal epithelium and its impact on gut homeostasis Current Opinion in Gastroenterology 34:413-419. [Epub 2018 Aug 29].
Cummings, R. J., Barbet, J., Bongers, G., Hartmann, B.M., Gettler, K., Muniz, L., Furtado, G.C., Cho, J., Lira, S.A.* and Blander, J.M.* (2016) Different intestinal phagocytes sample apoptotic cells in situ to orchestrate distinct programs of tissue homeostasis Nature 539:565-569. *co-corresponding authors. [Epub 2016 Nov 9].
