
Research
Colorectal cancer (CRC) is a major cause of cancer mortality worldwide, with poor survival rates despite improvements in systemic treatment. An emerging notion in the CRC field is that a stem-mesenchymal phenotype of the tumor epithelium, together with an immunosuppressive and desmoplastic stroma, predicts adverse outcomes in CRC patients better than the presence of prevalent mutations. Instead, key features of the tumor microenvironment, such as lack of T cell infiltration, increased immunosuppression, and a highly mesenchymal phenotype with desmoplastic stroma, predict adverse outcomes in CRC patients. Indeed, these are defining characteristics of the poor prognosis CMS4 subtypes, according to the most recent classifications of transcriptomic subtypes of CRC, which account for approximately 30% of all CRC cases. Therefore, considering their poor prognosis and resistance to therapy, the identification of the molecular mechanisms central to the acquisition of the mesenchymal phenotype of CRC tumors and establishing how that drives their aggressiveness is a fundamental question whose resolution will contribute to a better understanding of the etiopathogenesis of this type of poor-prognosis CRC.
Figure 1 A human colorectal tumor
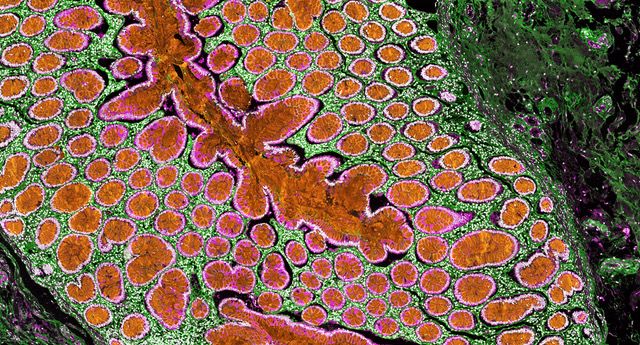
Our laboratory recently found that the expression of atypical protein kinase Cs, PKCz, and PKCl/i, is reduced in human mesenchymal CRC with serrated carcinoma and desmoplasia features that transcriptionally conform to the CMS4 subtype. The simultaneous inactivation of both kinases in the mouse intestinal epithelium results in spontaneous serrated tumorigenesis that progresses to advanced cancer with a strongly reactive and immunosuppressive stroma. Using this unique mesenchymal CRC mouse model and interrogation of human samples and datasets, we investigate two major questions: (1) what are the mechanisms of initiation that control and promote preneoplasia? and (2) what mechanisms drive therapy resistance and liver metastasis?
Figure 2 Extracellular hyaluronan (green) supporting the growth of mesenchymal Colorectal Cancer.
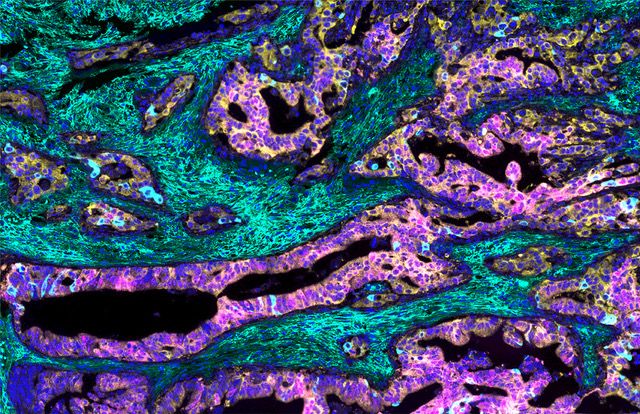
Current Projects:
- Intestinal inflammation and cancer
- Mechanisms of initiation in preneoplasia
- Therapy resistance in colorectal cancer
- Stromal activation
- Liver metastasis
Bio
Jorge Moscat is a Homer T. Hirst III Professor of Oncology in Pathology and Vice-Chair for Experimental Pathology at the Weill Cornell Medical College. Before joining Cornell, Jorge has served as Deputy Director of the Cancer Center and as the Institute’s Director of Metabolism Initiatives at the Sanford Burnham Prebys Medical Discovery Institute in La Jolla (California). Before that, Jorge was Chair of the Department of Cancer and Cell Biology and Associate Director of the Cincinnati Cancer Consortium at the University of Cincinnati Medical College, and Director of the Institute for Molecular Biology in the National Research Council of Spain.
Distinctions:
- Elected Member of the Academia Europaea (2006)
- Associate Director, University of Cincinnati Cancer Center (2008)
- Associate Director of Basic Research/ NCI-Cancer Center SBP Medical Discovery Institute (2015)
- Deputy Director/ NCI-Cancer Center SBP Medical Discovery Institute (2017)
- Chair of Tumor Cell Biology Study Section, NCI/NIH (2018)
- Homer T. Hirst III Professor of Oncology (2021)
