Research
Understanding how and why tissue regeneration occurs can shape therapeutic strategies for many human diseases. Zebrafish regenerate tissues like an injured heart or lost appendages. By studying heart regeneration in zebrafish, we aim to understand how innate tissue regeneration is regulated at the cellular and molecular levels. We will focus on the epicardium, the outermost layer of the heart, to:
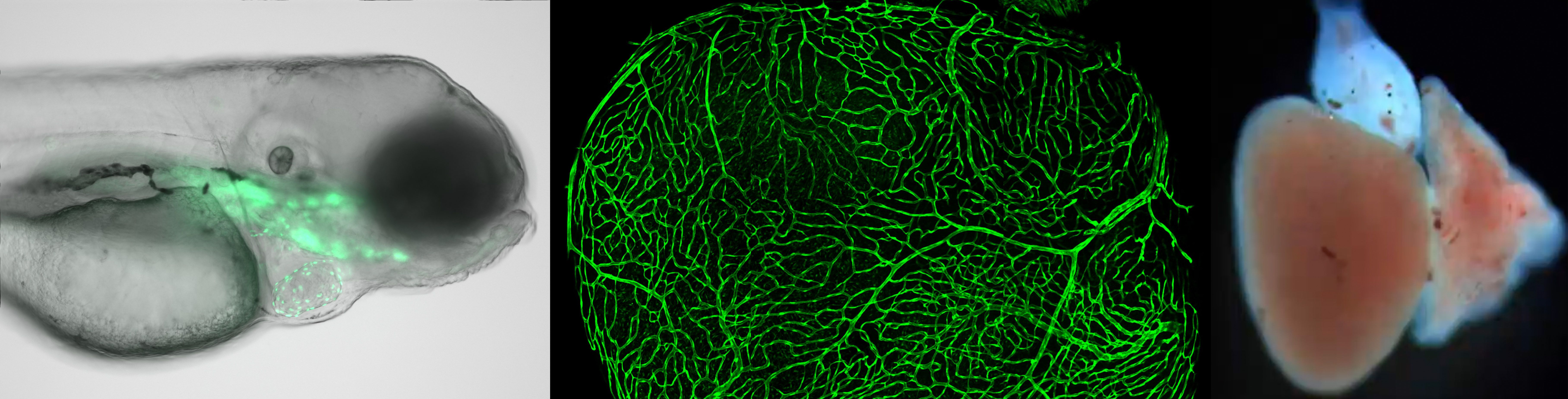
1) Dissect the contributions of epicardial progenitor cells to heart regeneration. The failure of the human heart to regenerate after a heart attack is one of the most significant challenges in regenerative medicine. While most studies focus on the heart muscle, less effort has been made to dissect the critical environment, or niche, provided by non-muscle tissues, such as the epicardium. We have found that the epicardium is essential to successful heart regeneration. We investigate how epicardial progenitor activation, paracrine secretion, and differentiation are regulated at the single-cell level to support heart muscle regeneration. Our study may ultimately inform approaches for activating the epicardial progenitors to enhance the limited regeneration displayed in humans after a heart attack.
Figure 1
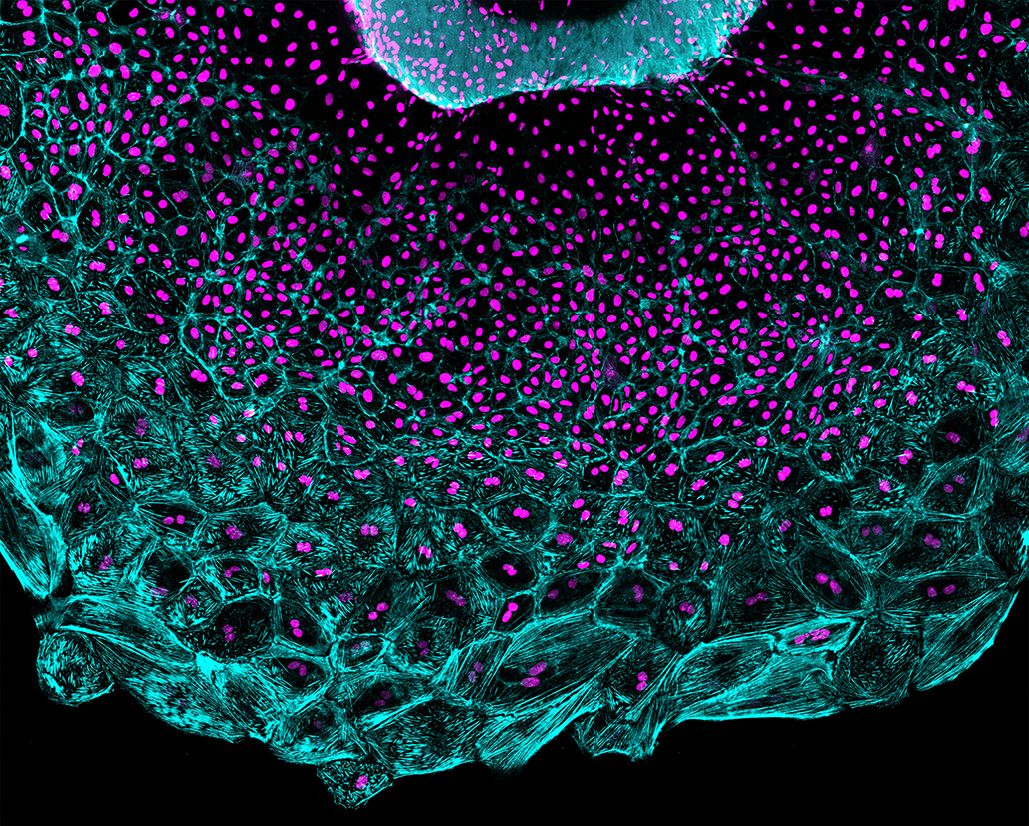
2) Uncover how cell cycle dynamics and polyploidization (greater than one genome) are regulated to engage in tissue regeneration. Polyploidization has been invoked in mechanisms of tissue regeneration, but the contributions and mechanisms are not well understood. We found a novel type of tissue regeneration of the zebrafish epicardium, in which cell cycle decisions between division and polyploidization are spatiotemporally regulated by cellular tension. We are investigating how cell cycle decisions are made and how and why polyploidy arises to participate in regeneration. Knowing this will provide ways to improve the regenerative capacities of tissues. Moreover, polyploid cells are present in normal tissues, such as the mammalian myocardium, and in pathological processes like cancer. Therefore, our study will yield insights into the biological implications of polyploidy in tissue development, homeostasis, and disease.
Figure 2
Overall, our work may ultimately help form the basis for the therapeutic repair of human tissues with inadequate regenerative capacity, like the heart.
Current projects:
- Epicardial progenitor cells in heart development and regeneration
- Spatiotemporal regulation of polyploidy in tissue regeneration
Bio
Jingli obtained his Ph.D. degree in Cell Biology from the Chinese Academy of Sciences in 2012. He then joined Dr. Kenneth Poss’s Laboratory at Duke University as a postdoctoral fellow. He was appointed as an Assistant Professor in the Cardiovascular Research Institute and Department of Cell and Developmental Biology at Weill Cornell Medical College in 2018.
Distinctions:
- President Award of the Chinese Academy of Sciences (2012)
- Outstanding Graduate Award of the Chinese Academy of Sciences (2012)
- American Heart Association Postdoctoral Fellowship Awards (2014, 2016)
- American Heart Association Career Development Award (2018)
- Mandel Fellow Award (2018)
