
Research
Cancer cells employ different strategies to evade the immune system, including activating inhibitory checkpoint molecules like PD-1 and CTLA-4 in cytotoxic T cells, or losing immunogenic antigens. Immune-based therapies, enhancing anti-tumor immunity, are frontline treatments for many cancers due to their striking clinical efficacy. However, relapse remains a challenge. How do tumors become resistant to immunotherapies? Our lab seeks to address this, focusing on melanoma as a model and translating our knowledge to other tumors, including lung, breast, and bladder cancers. Our previous research has uncovered prominent roles for myeloid-derived suppressor cells (PMID: 27828943), regulatory T (Tregs) cells, and tumor metabolism, specifically glycolysis (PMID: 33588426) and tryptophan catabolism (PMID: 32782249), in limiting the efficacy of immune checkpoint blockade. We are further exploring how metabolic pathways and microbiota-derived metabolites impact on immunotherapy response.
Other major interest is tumor antigenicity. Tumor cells evade immune recognition by losing antigens, becoming “immune escape variants” driving resistance. Our group has discovered that T cell co-stimulation through OX40 induces neutrophil infiltration, which eliminates “immune escape variants” (PMID: 37001503), prompting us to explore neutrophil mobilization strategies. We are also developing approaches to increase tumor antigenicity, like delivering tumor antigens via commensal bacteria, and predict how functional T cell repertoires can inform of immunotherapy responses and help design tumor vaccines. Additionally, we are monitoring how antigen stimulation induces differentiation of single-to-double positive T cells with polyfunctional characteristics (PMID: 35604411), which will aid identifying antigen-specific T cells and pathways underlying their functional plasticity and clinical relevance.
Advancing strategies for enhancing immunotherapies is a key goal. We are investigating the efficacy of combining T cell co-stimulation, chemotherapy, and targeted therapies with checkpoint blockade. Our previous work has revealed that cyclophosphamide with GITR-mediated T cell co-stimulation induces immunogenic tumor cell death, expanding oligoclonal, tumor-infiltrating cytotoxic T cells (PMID: 34676831). We are now examining cyclophosphamide and checkpoint blockade combo efficacy. We have also demonstrated that RAF/MEK inhibition with CTLA-4 blockade enhances T cell function in RAS pathway mutant tumors (PMID: 24416731; PMID: 30995478) and are currently evaluating mutant KRAS inhibitors with immunotherapy for lung cancers. Furthermore, we are engineering chimeric antigen receptor (CAR) T cells for melanoma, glioblastoma and prostate cancer and evaluating their therapeutic potential with combination strategies targeting the immunosuppressive tumor microenvironment.
Figure.
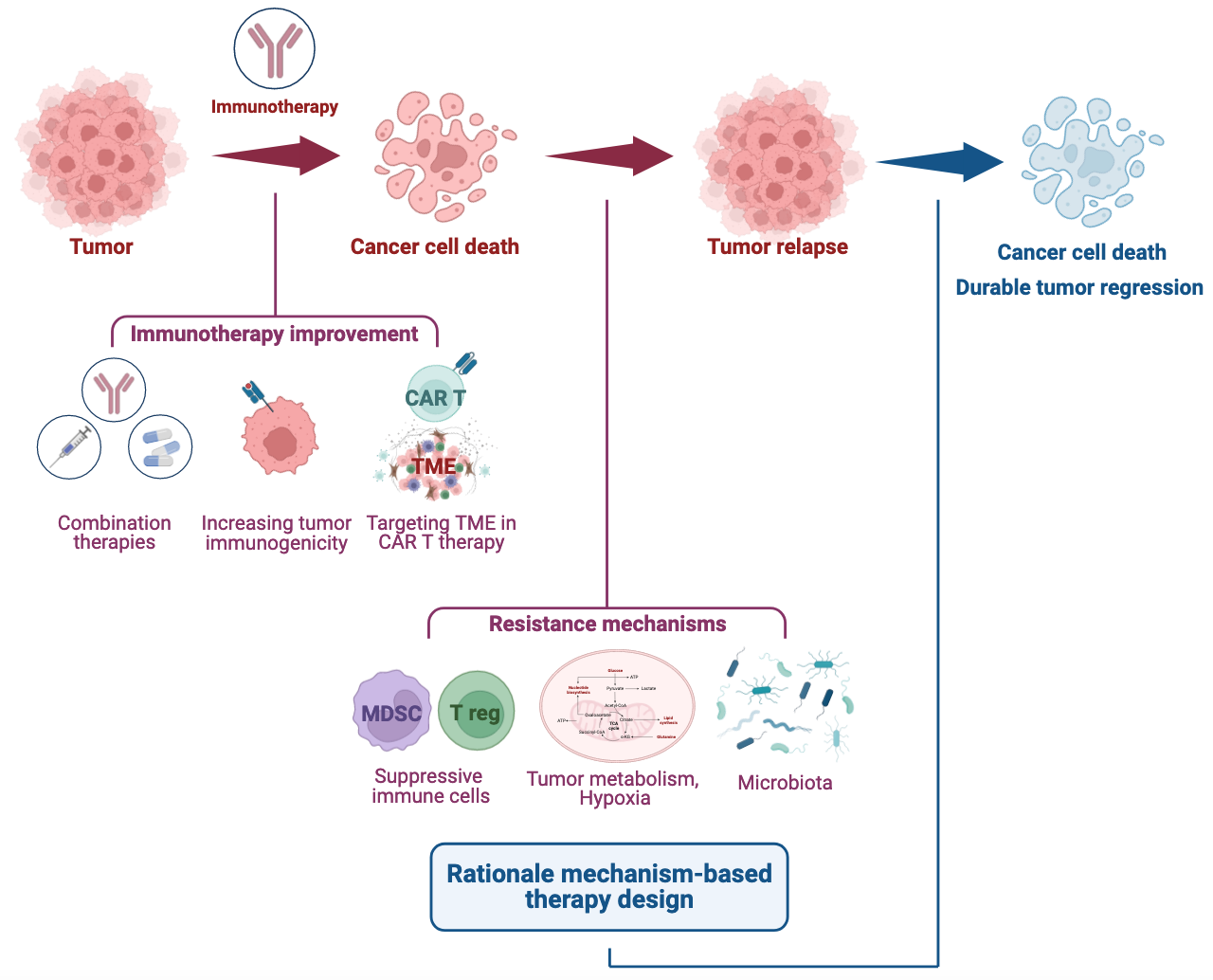
We hope our findings enable us to initiate clinical trials leveraging the potential of the immune system for effectively managing and eradicating solid tumors.
Current Projects:
- Myeloid derived suppressor cells and regulatory T cells in tumor immunity.
- Impact of tumor metabolism on immunotherapy response.
- Tumor antigenicity and approaches to predict antigen-specific, functional T cells.
- Optimal cancer vaccine strategies in pre-clinical mouse models.
- New strategies combining immunotherapy with chemotherapy and targeted agents.
- Leveraging T cell co-stimulation to improve immunotherapies.
- Targeting the tumor microenvironment to improve CAR T cell therapy.
Bio
I received his BA degree from Princeton University and my MS, PhD and MD degrees from New York University. I am the Meyer Director of the Sandra and Edward Meyer Cancer Center and Professor of Medicine at Weill Cornell Medicine. I am a clinician-scientist exploring innovative immunotherapeutic strategies in laboratory models, and a principal investigator in numerous pivotal clinical trials. I helped establish immunotherapy as a standard approach to cancer treatment and was instrumental in the clinical development leading to the approval of ipilimumab and the combination of nivolumab and ipilimumab for advanced melanoma. I supervise an NIH R01-funded basic science laboratory that is focused on investigating novel immunotherapeutic agents in pre-clinical laboratory models. The focus of my translational research laboratory is to investigate innovative means to modulate the immune response to cancer as well as to better understand the mechanistic basis for sensitivity and resistance to currently available immunotherapies.
Distinctions.
I have received honors including: American Association for Cancer Research (AACR) Richard and Hinda Rosenthal Memorial Award, the Giants of Cancer Care in Melanoma Award, the Berson Alumni Achievement Award in Clinical and Translational Science and the Zelmanovich Young Alumni Achievement Award from NYU Grossman School of Medicine, the Alfred Taubman Prize for Excellence in Translational Medical Research (Univ of Michigan) and has been designated a Fellow of the American Society of Clinical Oncology (FASCO), as well as board of directors.
I have been awarded the Distinguished Alumni Award at MSK, the AACR-Joseph H. Burchenal Award for Outstanding Achievement in Clinical Cancer Research, the ESMO Award for Immuno-Oncology and the David Karnofsky Award from ASCO. Dr. Wolchok has served on the Board of Directors of ASCO and the Society for Immunotherapy of Cancer (SITC) and was the Treasurer of SITC. I am an elected member of American Association for Cancer Research (AACR) board of directors, elected to the AACR academy of fellows (FAACR), fellow of the academy of Immuno-Oncology (FAIO), the American Society for Clinical Investigation (ASCI), Association of American Physicians (AAP) and am Chair of the Melanoma Committee for the ECOG-ACRIN NCI cooperative group. Most recently I has been elected to the National Academy of Medicine (NAM). I am a full member of the Ludwig Institute for Cancer Research and center director for the Parker Institute of Cancer Immunotherapy.
I am a full member of the Ludwig Institute for Cancer Research and center director for the Parker Institute of Cancer Immunotherapy. A former Damon Runyon-Lilly Clinical Investigator, and now serve as a member of the Damon Runyon Clinical Investigator Award Selection Committee and have mentored three Damon Runyon Awardees.
