
Research
Vascular system development
A functional vascular system is crucial for the development of the mammalian embryo. We examine the molecular and genetic pathways that regulate the three principal processes of vascular endothelial development: endothelial cell lineage determination, vasculogenesis and angiogenesis. A combination of molecular and genetic approaches, using embryonic stem cells, primary endothelial cells and mouse models are employed in these studies. The groundwork for our research program includes endothelial-specific genes that we identified in a genetic expression screen, gain- and loss-of function mutant mouse strains, and a reporter mouse strain in which cells of the endothelial cell lineage express a fluorescent marker. Present projects examine the functions of the endothelial gene Egfl7 in endothelial progenitor cells, during endothelial-to-hematopoietic transition, and in preeclampsia.
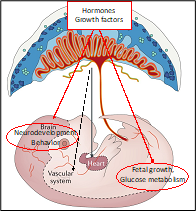
Placental development and disease
The placenta is a vital organ at the interface between mother and fetus during gestation. It is crucial for the exchange of gases and nutrients, the production of pregnancy-specific hormones, and for immune protection of the embryo. Impairment of placental function, including defects in placental genes or environmental insults such as virus infection, developmentally program the fetus for chronic disease later in life such as cardiovascular disease, diabetes and obesity. This phenomenon is termed fetal programming or developmental origins of adult health and disease. We are using in vitro cell cultures, ex vivo human placental explant cultures and organoids, and mouse models to study how mutations or viral insults impact on placental development and how this affects the health of the fetus and offspring. One ongoing project investigates the molecular pathways triggered by SARS-CoV-2 infection during pregnancy and the resulting inflammatory responses observed in the placenta. A second project investigates the role of a microRNA that is expressed in the placenta in a sex-specific manner and whose loss-of function results in placental defects in mice. Present studies focus on dissecting molecular and epigenetic links between the role of the microRNA during placentation and neuronal development and glucose metabolism in the fetuses and in adults.
Current Projects:
- Endothelial lineage development
- Placental insufficiencies and fetal programming
Bio
Dr. Stuhlmann joined Weill Cornell Medicine in 2006 as Professor of Cell and Developmental Biology. She received a doctorate in Biology in 1983 from the University of Hamburg, Germany, under the mentorship of Rudolf Jaenisch. She conducted her post-doctorate training with Richard Mulligan at the Whitehead Institute and MIT, and with Paul Berg at Stanford University School of Medicine. Prior to joining Weill Cornell, Dr. Stuhlmann held faculty positions as an Assistant Professor at Mt. Sinai School of Medicine in New York City (1991-1999), and as an Associate Professor at The Scripps Research Institute in La Jolla, CA (1999-2006).
Distinctions:
- Basil O’Connor Starter Scholar Research Award, March of Dimes Foundation
- Harvey Klein Endowed Professorship of Biomedical Sciences, WCM
- Adjunct Professor, Department of Biomedical Sciences, College of Veterinary Medicine at Cornell University
- Invited Professorship, University of Rome Tor Vergata, Rome, Italy
- Program Director, T32 Training Program in Developmental and Stem Cell Biology
- Co-Chair, Cell and Developmental Biology program, Weill Cornell Graduate School of Medical Sciences
