
Research
The Hollis lab has four main areas of focus that span from basics of circuit function to clinical interventions. We are interested in: 1) the function and modulation of neuronal circuits underlying movement; 2) the response of neural architecture to spinal cord injury; 3) the recovery of central and peripheral neuronal circuits after injury; and 4) molecular control over glial interactions after central and peripheral injury. Our studies use genetic, molecular, and behavioral tools along with optogenetic or chemogenetic modulation of, and optical recording from, neuronal networks in order to understand mechanisms of injury and recovery.
Central and peripheral nervous system injuries result in the disruption of neuronal circuits, inflammation, glial responses, Wallerian degeneration, and varying degrees of recovery, depending on the location of injury. Within the spinal cord, secondary degeneration, scarring, and a reduced intrinsic growth capacity limit regeneration and recovery. In contrast, peripheral regeneration can be much more robust, with remarkable restoration of function in rodent models of nerve injury. Both central and peripheral injuries disrupt the function of sensorimotor circuits. After spinal cord injury, a limited amount of endogenous recovery is possible, likely driven by the plasticity of neural circuits in close proximity to the lesion site or from compensatory function from supraspinal projections. The cortical mechanisms supporting this recovery are not well understood, nor is it known whether limitations on plasticity of cortical networks underlie the failure of current therapies to restore function lost to spina cord injury.
Our goal is to develop novel therapeutic interventions that take advantage of circuit plasticity to promote recovery from neurological injury. To do so, we are investigating all levels of the circuits supporting movement. Within the spinal cord, we are studying developmental regulation of axon regeneration, as well as mechanisms of the astrocyte response to injury to limit secondary degeneration and maintain local circuit survival. In the periphery, we are targeting pro-regenerative pathways and neuroimmune interactions to improve surgical interventions that support the return of hand function in individuals living with chronic cervical spinal cord injury. Within the cortex, we are focused on recovery and plasticity of the sensory and motor networks required for interpreting and regulating skilled movement and motor learning.
Figure 1. Learning of precision motor control shapes corticospinal motor networks. a) Illustration of retrograde transduction of corticospinal neurons. b) Illustration of head-fixed behavior during imaging. c) Success rate on isometric pull tasks at three distinct training phases. Mice were proficient on a simple, adaptive isometric pull early in training, whereas mice had to learn our skilled, precision isometric pull task. d) Successful task execution is impaired on precision, but not adaptive, isometric pull following bilateral pyramidotomy. e) Correlation coefficients plotted in heatmaps where each point is the coefficient from a neuron pair. There is more widespread network activation in adaptive pull with pairs of neurons showing higher correlation in activity during movements. Precision coefficients are smaller indicating diverse activity of individual neurons during movement.
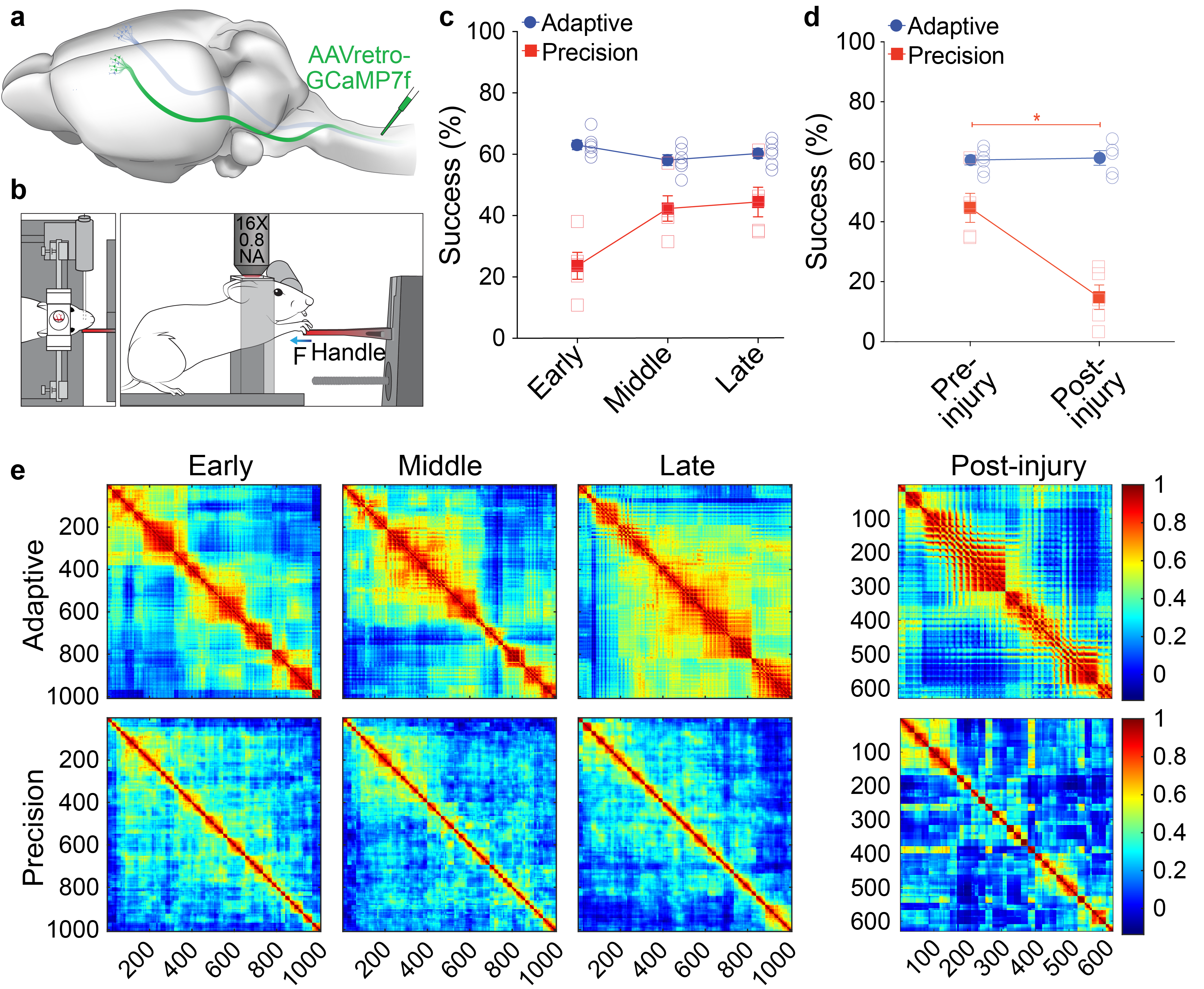
Figure 2. Sciatic nerve crush triggers macrophage interactions with regenerating sensory neurons in the dorsal root ganglia (DRG). a) Macrophages labeled with Iba1 antibody in iDisco cleared L4 DRGs from intact, 3-, and 7-days post-injury. b) IMARIS 3D reconstructions show macrophage contacts with regenerating sensory neurons labeled with retrograde tracer CTB at 7 days after nerve crush.

Current Projects:
- Motor control
- Sensorimotor networks
- Spinal cord injury
- Neuroimmune interactions
- Reactive astrogliosis
- Clinical trial in tetraplegia
Bio
Edmund Hollis, PhD, obtained his bachelor’s degree in biomedical engineering from the University of Southern California. He performed his graduate and postdoctoral training at the University of California, San Diego with Mark H. Tuszynski, MD, PhD and Yimin Zuo, PhD, respectively. He became Director of the Circuit Repair Laboratory at the Weill Cornell Medicine affiliated Burke Neurological Institute in 2015 and an Assistant Professor of Neuroscience in 2016. He has received several research awards, including a 2017 NIH Director’s New Innovator Award. Currently Dr. Hollis serves as the Postdoctoral Advancement Advisor and IACUC representative at BNI.
Distinctions:
- NIH Director’s New Innovator Award 2017
Selected Publications:
Serradj N, Marino F, Moreno-López Y, Bernstein A, Agger S, Soliman M, Sloan AM, and Hollis E. (2023) Task-specific modulation of corticospinal neuron activity during motor learning in mice. Nat Comms. 14: 2708.
Li Y and Hollis E. (2023) Nicotinic signaling is required for motor learning but not for rehabilitation from spinal cord injury. Neural Regen Res. 18: 364-367.
- Li Y and Hollis E. (2021) Basal forebrain cholinergic neurons selectively drive coordinated motor learning in mice. J Neurosci. 49: 10148-10160.
