
Research
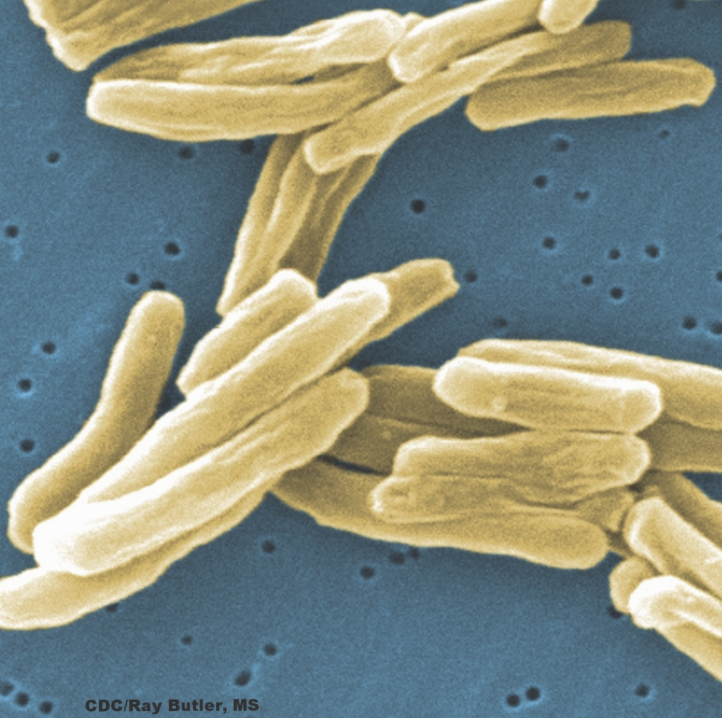
Our projects today pursue the following topics: host pathways controlling susceptibility and resistance to Mycobacterium tuberculosis (Mtb); the genes on which Mtb depends to survive the stresses associated with aerosol transmission; the biology of Mtb in non-replicating (NR) states, during which the pathogen displays phenotypic resistance to conventionally developed antibiotics; efforts to overcome Mtb’s phenotypic resistance by identifying the genes on which it depends and developing new antimicrobial agents; and inhibition or exploitation of protein degradation and proteostasis pathways in bacteria, protozoa, cancer cells and cells of the immune system.
In Mtb, we are targeting two “hub” enzymes—one enzyme (lipoamide dehydrogenase) that participates in multiple functionally distinct complexes and another (phosphopanthetheinyl transferase) that controls the activation of multiple functionally distinct pathways—as well as nicotinamide adenylyl transferase, regarded as one of the most highly “vulnerable” enzymes in Mtb. Discovery of the mycobacterial proteasome and its contribution to Mtb’s resistance to host-derived reactive nitrogen species led to the development by Gang Lin and colleagues of the first species-selective proteasome inhibitors, followed by species-selective inhibitors for Plasmodium falciparum and non-cytotoxic inhibitors of the human immunoproteasome that spare the constitutive proteasome. With Franck Barrat at HSS and others, we are studying their promise for trating autoimmune and inflammatory disorders.
These campaigns call on disciplines ranging from immunology and microbiology to biochemistry and medicinal chemistry. Reflecting that, the lab is multi-disciplinary and internally and externally collaborative. Those features apply to the entire Global Health Floor in the Belfer Building, which our lab shares with the labs of Sabine Ehrt, Dirk Schnappinger, Dan Fitzgerald, Kyu Rhee, Kohta Saito and Kate Dupnik. We collaborate as well with Jean-Laurent Casanova and Jeremy Rock at Rockefeller and with Michael Glickman at MSKCC. Still other collaborations with academic partners, pharmaceutical companies and the Global Alliance for TB Drug Development are supported by the Bill & Melinda Gates Foundation’s TB Drug Accelerator.
Figure 1
Current Projects:
- Biology of aerosol transmission of M. tuberculosis (Mtb)
- Biology of Mtb-macrophage interactions
- Drug development for tuberculosis, malaria and cancer
Bio
Carl Nathan graduated from Harvard College and Harvard Medical School and trained in internal medicine and oncology at Massachusetts General Hospital, the National Cancer Institute and Yale. He joined the faculty at Rockefeller University for 10 years before moving to Weill Cornell Medicine as Stanton Griffis Distinguished Professor of Medicine. There he served as founding director of the Tri-Institutional MD-PhD Program, acting dean of the Medical College, dean of the Weill Cornell Graduate School of Medical Sciences, and chair of the Department of Microbiology and Immunology. He co-chairs the editorial board of the Journal of Experimental Medicine and serves on the boards of the Proceedings of the National Academy of Sciences and Science Translational Medicine.
Distinctions:
- National Academy of Sciences
- National Academy of Medicine
- American Academy of Arts and Sciences
- American Academy of Microbiology
- Koch Prize
- Sanofi-Institut Pasteur Senior International Scientist Award
- Weill Exemplary Achievement Award
