
Research
What are the main iRhom/ADAM17-dependent signaling pathways?
Research in our lab is focused on the cell surface metalloprotease ADAM17 (a disintegrin and metalloprotease 17) has a crucial role in regulating several major medically relevant signaling pathways, including TNFa, IL-6R and EGFR-signaling. The TNFa and IL-6R signaling pathways are established targets for treatment of autoimmune diseases, and EGF-receptor signaling, which is important for normal development and adult homeostasis, but can also promote cancer and autoimmune disease. ADAM17 is controlled by its essential partners, the seven membrane-spanning inactive Rhomboid proteins iRhom1 and 2. Together with the iRhoms, ADAM17 acts as a set of signaling scissors that cleave and release membrane proteins from cells. This process, referred to as “protein ectodomain shedding”, can activate or inactivate the substrate protein, or substantially change its functional properties. Dysregulation of iRhom/ADAM17 signaling can cause Rheumatoid Arthritis, Systemic Lupus Erythematosis-Glomerulonephritis and other diseases such as neuroinflammation and defects in heart valve development. ADAM17 can be rapidly and post-translationally activated by a variety of signaling pathways, and the iRhoms have emerged as essential regulators of ADAM17 and of its substrate targeting. Work in our lab set the stage for the foundation of the biotech startup SciRhom, which has developed novel function blocking antibodies against iRhom2 in order to improve the treatment of patients suffering from autoimmune diseases. Our current knowledge of the iRhom/ADAM17 complex is just the tip of the iceberg, and many exciting questions remain regarding the regulation and function of these fascinating molecules, such as how they target and process their substrates, how these functions are controlled, and what role the iRhom/ADAM17 signaling hub has in development, autoimmune diseases, neuroinflammation and cancer.
Figure 1
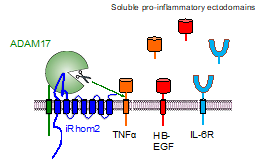
Current Projects:
- Role of iRhom2/ADAM17 in efferocytosis, a protective anti-inflammatory process
- Role of iRhom2/ADAM17 in the olfactory epithelium
- iRhom2/ADAM17 in autoimmune diseases such as RA
- Regulation of ADAM17 by iRhom1
- Identification of novel regulators of iRhom/ADAM17 signaling
Bio
Carl Blobel obtained his MD degree from the Justus-Liebig University in Giessen, Germany, and a PhD in Biochemistry and Biophysics from UCSF. He is currently a Senior Scientist and Program Director of the Arthritis and Tissue Degeneration Program at the Hospital for Special Surgery, where he holds the Virginia F. and William R. Salomon Chair in Musculoskeletal Research. He is also Professor of Medicine and of Biochemistry, Cellular and Molecular Biology at Weill Cornell Medicine.
Distinctions:
In 2015, Dr. Blobel was elected to the Association of American Physicians in recognition of his outstanding contributions to medicine. In 2017, he was awarded a Distinguished Affiliated Professorship by the Technical University of Munich in 2017, which recognizes "researchers of international prominence who have not only significantly shaped their own discipline but have also inspired other areas within the scientific community." Dr. Blobel has over 15,750 citations in the Web of Science Core collection ISI citation report and an h-factor of 70 (July 2023).
