
Research
The Tulpule Lab studies how the compartmentalization of oncogenic fusion proteins can both co-opt and disrupt cellular condensates in order to drive cancer. We have two main areas of interest.
1. Oncogenic kinase fusions and signaling from RTK protein granules
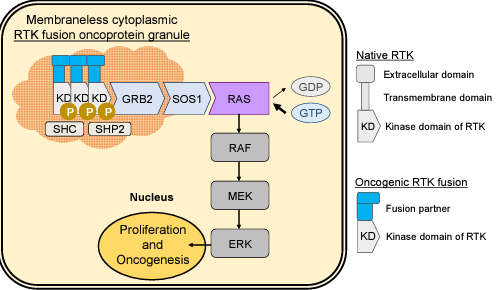
Receptor tyrosine kinase (RTK)-mediated activation of downstream effector pathways such as the RAS/MAPK signaling cascade was thought to occur exclusively from lipid membrane compartments in mammalian cells and cancer. Yet the majority of chimeric (fusion) oncoproteins involving RTKs such as ALK or RET retain the intracellular kinase domain but lose the extracellular and transmembrane sequences, presenting fundamental questions as to how and where these oncoproteins organize RTK and RAS signaling in cells. We recently discovered that multiple prominent RTK fusion oncoproteins including EML4-ALK and CCDC6-RET do not localize to lipid membranes, but instead undergo de novo higher-order assembly into membraneless cytoplasmic protein granules that coordinate lipid-membrane independent RAS activation and MAPK signaling. These data establish a new pathogenic subcellular structure in cancer: membraneless RTK protein granules. Ongoing work in the laboratory aims to build a detailed molecular understanding of the signaling properties of these new biomolecular condensates and test whether pharmacologic disruption of RTK protein granules can be translated into a new therapeutic strategy for this class of cancers.
Figure 1
2. Oncogene disruption of cellular DNA repair networks
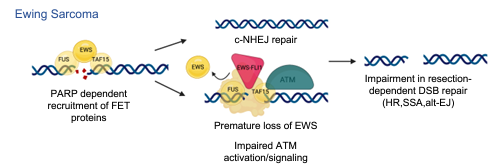
While oncogenes promote cancer cell growth, unrestrained proliferation represents a significant stressor to cellular homeostasis networks such as the DNA damage response (DDR). To enable oncogene tolerance, many cancers disable tumor suppressive DDR signaling through genetic loss of DDR pathways and downstream effectors (e.g., ATM or p53 tumor suppressor mutations). Whether and how oncogenes can help “self-tolerize” by creating analogous functional deficiencies in physiologic DDR networks is not known. Our work focuses on the FET family of intrinsically disordered proteins (FUS, EWS, TAF15) that are frequent oncogenic fusion partners in a diversity of pediatric sarcomas and leukemias. Using Ewing sarcoma as a model of FET rearranged cancers, we recently discovered that the EWS-FLI1 fusion oncoprotein is recruited to sites of DNA damage and interferes with native FET protein function in activating the DNA damage sensor ATM. These data establish functional ATM deficiency as the principal DNA repair defect in Ewing sarcoma and the compensatory ATR signaling axis as a collateral dependency and therapeutic target in multiple FET rearranged cancers. Our ongoing work on Ewing sarcoma and other pediatric FET fusion sarcomas aims to characterize the mechanisms by which FET fusion oncoproteins disrupts DNA repair with a focus on phase separation by disordered native FET proteins, as well as testing novel therapeutics to exploit this vulnerability in xenograft models and ultimately patients.
Figure 2
Current Projects:
- Mechanism of RAS/RAF activation at membraneless RTK granules
- Components and assembly properties of RTK granules
- Chemical disruption of pathogenic RTK granules in lung cancer
- Disordered native FET proteins in DNA repair condensates
- FET fusion oncoprotein interactions with DNA repair machinery
- Testing ATR inhibitors in pediatric sarcoma xenografts and patients
Bio
Asmin received his B.A. from Princeton University (2002) in Molecular Biology with a Certificate in Biophysics. He completed his PhD in Dr. George Daley’s lab at Boston Children’s Hospital and received his MD from Harvard Medical School. After his MD/PhD studies, Asmin completed Pediatric Residency and Pediatric Hematology/Oncology Fellowship at UCSF. His postdoctoral work was performed in Dr. Trever Bivona’s lab studying Ewing sarcoma and FET fusion oncoprotein biology. Asmin launched his own independent research group in 2017 as a UCSF Physician-Scientist Scholar and moved to NYC in 2022 to join the faculty at MSKCC and Cornell.
Distinctions
- NCI Career Development Award K08 (2023 – 2028)
- Hyundai Hope on Wheels Young Investigator Award (2023-2025)
- Sarcoma Center Award, Memorial Sloan Kettering Cancer Center (2023)
- St. Baldrick’s Foundation Scholar Award (2018)
- UCSF Physician-Scientist Scholar Program (2017-2022)
- Outstanding Performance and Accomplishment in Research, Harvard Medical School (2011)
