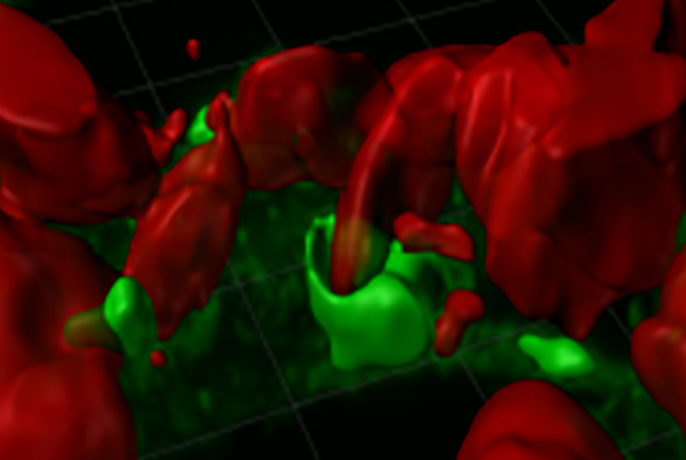
Immune cells in the brain called microglia can partially break down large amyloid plaques characteristic of Alzheimer’s disease by latching on to them, forming a sort of external stomach and releasing digestive enzymes into the space, according to a preclinical study by Weill Cornell Medicine investigators. The findings could ultimately lead to therapies that boost the ability of microglia to break down amyloid plaques.
The study, published Dec. 6 in Cell Reports, shows that the breakdown process, called digestive exophagy, may also help explain seemingly contradictory reports that microglial cells can spread plaques in Alzheimer’s.
Microglia are scavengers that move around the brain, consuming small bits of cellular trash like microbes, dead cells and debris. They do this by wrapping themselves around the substance and encapsulating it in a vesicle. The vesicle then ferries the cargo to a membrane-bound organelle within the cell called a lysosome that is filled with digestive enzymes. Researchers suspected that microglia could also break down amyloid plaques, but it was unclear how the cells could consume these massive aggregates, which are much bigger than they are.
“We found that the cells basically attach a lysosome onto a large plaque, and they expel enzymes into the space that can digest the amyloid,” said Dr. Frederick Maxfield, the Vladimir Horowitz and Wanda Toscanini Horowitz Distinguished Professor in Neuroscience at Weill Cornell Medicine.
A Familiar Flavor
Thinking about how microglia could eat something very large in the brain reminded Dr. Maxfield of macrophages, which are immune cells that do a similar scavenging job throughout the rest of the body. In previous work, his lab found that when macrophages run into something too big to wrap around—such as a clump of lipoproteins in an atherosclerotic plaque—they form a kind of external stomach that digests the clump bit-by-bit with lysosomal enzymes.
To see whether microglia do the same thing, the team, led by Dr. Santiago Solé-Domènech, assistant professor of research in biochemistry and Dr. Rudy Jacquet, a Weill Cornell Graduate School of Medical Sciences doctoral student in the Maxfield lab at the time of the study, first turned to cultured mouse microglial cells.
In a series of experiments, they showed that when one of these cells touched a large amyloid plaque in a lab dish, it formed a partially sealed space around the aggregate, like macrophages do. “The microglial lysosomes secreted their contents, and they acidified the region to activate the enzymes that digest the amyloid deposits,” said Dr. Maxfield.
Next, the team analyzed a mouse model of Alzheimer’s disease. Co-author Dr. Marie-Ève Tremblay, a professor at the University of Victoria, used electron microscopy to image brain samples and saw pockets formed by microglial cells on plaques that are characteristic of digestive exophagy. She also observed a lysosomal enzyme in the space.
What Goes Down Must Come Up
Microglia degrade plaques, but they are also known to contribute to the formation of plaques. This paradox was intriguing, and the researchers wondered if digestive exophagy could play a role in spreading Alzheimer’s plaques to other parts of the brain.
In previous research, the team loaded cultured microglial cells with smaller bits of plaques, called amyloid fibrils. When the researchers did this, the cells took a long time to break them down in internal lysosomes, and they eventually spit out the difficult-to-digest pieces.
In the current study, the team also loaded microglia with amyloid fibrils, but they put the cells near big plaques. “We saw that the microglia would try to eat the plaques from the outside, but because they were already loaded with amyloid fibrils, they would secrete these smaller pieces toward the plaque,” said Dr. Sole-Domenech.
Microglia cells can move quickly throughout the brain, so they can easily move to a different location after digesting a plaque. “So, if microglial cells start to do digestive exophagy on another large object, they could release those seeds of amyloid fibrils into a new area,” said Dr. Maxfield.
Toward a Treatment
Now that the team has observed digestive exophagy in mice, they will assess whether human cells also use this process. Human induced pluripotent stem cells can mature into many types of cells, including microglia, which would give the researchers the opportunity to conduct a wide range of experiments.
One area to investigate in the various models is how the new Food and Drug Administration-approved treatments for Alzheimer’s, which are antibodies, clear away plaques. “We are very interested in treating microglia with these antibodies to see how that affects digestive exophagy,” said Dr. Solé-Domènech.
Other existing drugs might be able to enhance microglial enzyme activity or boost the secretion of enzymes into the space. “We know various drugs increase lysosomal secretion in macrophages, and now we can test some of those in microglial cells as a starting point,” said Dr. Maxfield.
This work was supported in part by the National Institute on Aging and the National Heart, Lung and Blood Institute, both part of the National Institutes of Health, through grant numbers RF1AG078244 and R01HL093324. Additional support was provided by the Cure Alzheimer’s Fund, the Swedish Research Council International Postdoctoral Fellowship number 637-2013-503/D0050301, the Leon Levy Foundation Fellowship in Neuroscience, the Mexican Council of Science and Technology, and a Canada Research Chair (Tier 2) in Neurobiology of Aging and Cognition.
