
Research
With the advent of high-resolution cryo-electron microscopy (cryo-EM) there is now a great need for methods to study structural dynamics, and single molecule kinetics of proteins under close-to-native conditions. We develop new high-speed atomic force microscopy (HS-AFM) technology with millisecond temporal resolution to (i) study single molecule kinetics, (ii) conformational transitions and (iii) detect rare, transient conformational states, complementing our structural understanding of biomolecules, difficult to analyze using other methods. While these developments are useful and applicable to analyze any biomolecule, the research in our laboratory is focused on the investigation of membrane proteins, i.e., transmembrane channels and transporters. Using HS-AFM, we characterized the single molecule kinetics of the light-driven proton pump bacteriorhodopsin and of a glutamate transporter. The openness of the HS-AFM system, taking movies of molecules at ambient temperature and pressure, in physiological buffer solution and - in the case of membrane proteins - in membranes, allows to manipulate the environmental conditions (ions, pH, ligands, temperature, force) and monitor the molecules' reactions. Recently, owing to the ability of HS-AFM to take dynamic movies of single molecules, we discovered a short-lived non-canonical pentameric state of the TRPV3 channel. We often complement our HS-AFM analyses with cryo-EM structure determination and computational modeling or simulation. Together, these approaches open new avenues in biophysics / structural biology: The characterization of fast single molecule kinetics, the discovery of short-lived conformational states and conformational transitions, completing our understanding of transport processes through the membrane.
Figure 1
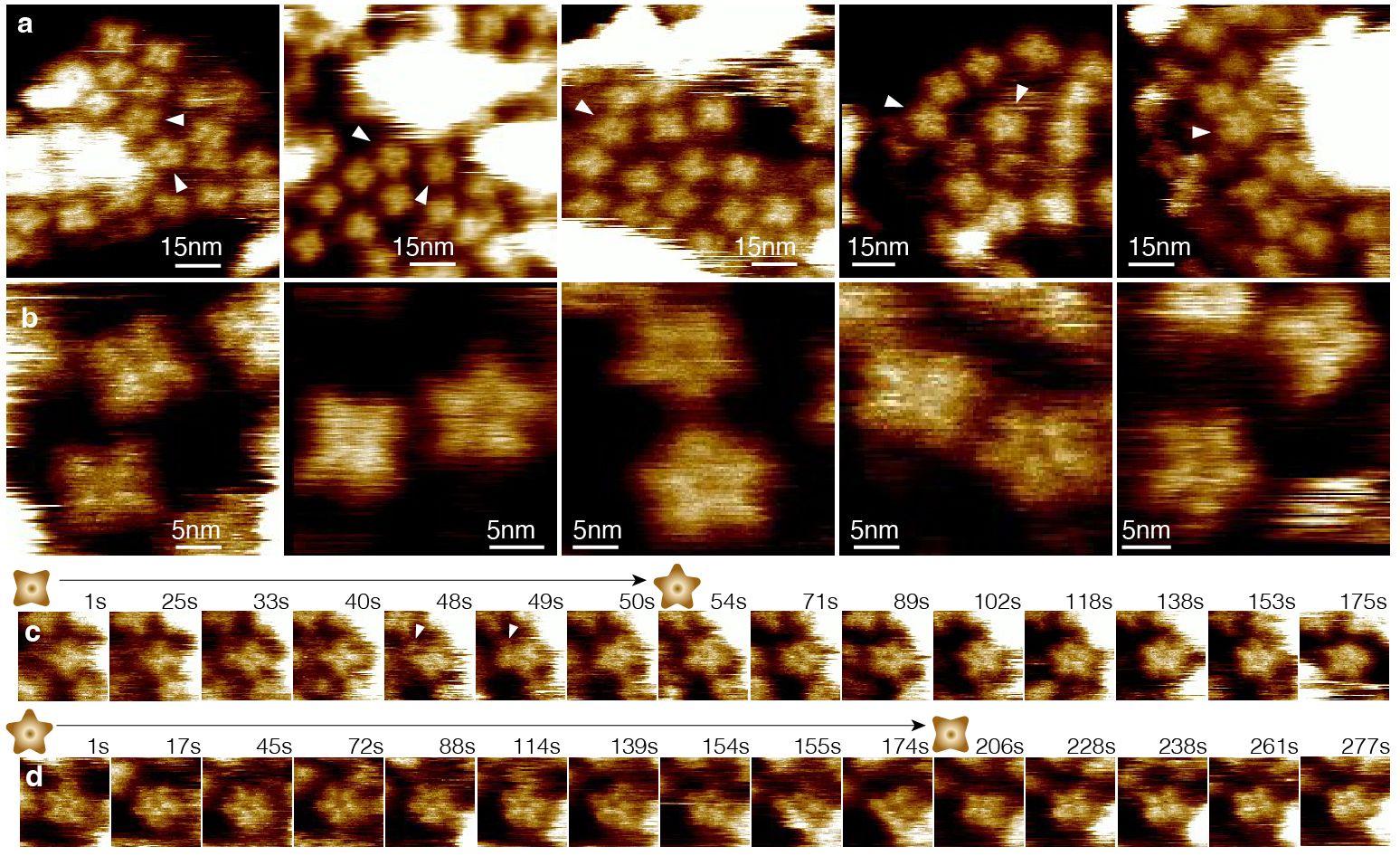
Figure 2
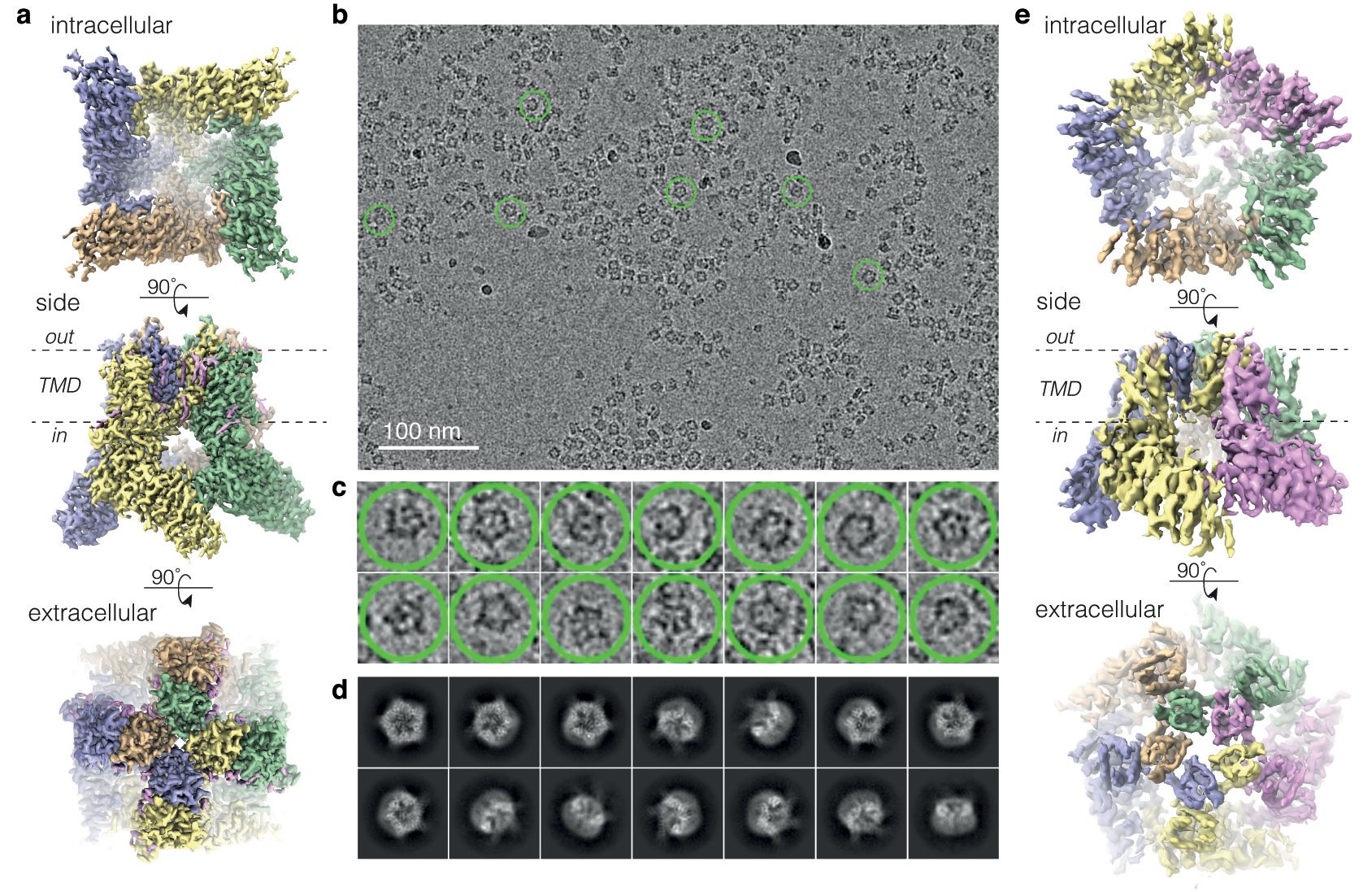
Current Projects:
- High-Speed AFM of rare and transient conformational states
- Single-molecule kinetics.
Bio
Born in Basel, Switzerland, Swiss, also naturalized in France. BS, 1995, MS, 1996, in Biology-II, and PhD in Biochemistry, 2001, at the Biozentrum, University Basel, Switzerland. Postdoctoral Fellow at the Biozentrum, University Basel, Switzerland, 2001, and the Institut Curie, Paris, France, 2001-2003. Chargé de Recherche (CR2), 2004-2007, and Directeur de Recherche (DR2) 2007-2012, INSERM, Institut Curie, Paris, France. Habilitation à Diriger des Recherches (HDR), Université Pierre et Marie Curie, 2005. Directeur de Recherche (DR1) INSERM, INSERM/Aix-Marseille Université, Luminy, Marseille, France, 2012-2016. Professor of Physiology and Biophysics in Anesthesiology, Weill Cornell Medicine, New York, USA, 2017-Now.
Distinctions:
- La Médaille de la Ville de Paris (Échelon Argent) 2007
- ERC Starting / Consolidator Grant 2012
- NIH Director's Pioneer Award (DP1) 2019
- W.M. Keck Foundation Award – Medical Research 2022
