
Research
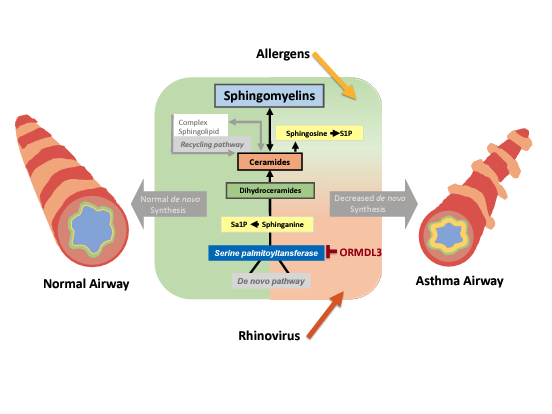
There is increasing evidence that sphingolipid metabolism is altered in asthma. ORMDL3 in the asthma susceptibility locus 17q21, the most replicated and most significant genetic asthma locus, is a critical regulator of sphingolipid de novo synthesis. The Worgall lab uses primary airway epithelial cells, mouse models and airway and blood cells and fluids from children with asthma and neonatal lung disease to study the mechanisms of how disordered sphingolipid synthesis leads to asthma. The lab found that decreased de novo sphingolipid synthesis results in airway hyperreactivity, the main feature of asthma, and that sphingolipid metabolism is altered in children with asthma.
The lab is currently investigating how viral infections, the most common trigger of asthma, shape the inflammatory and immune responses in relation to sphingolipid metabolism. Given the strong prevalence of asthma and the ORMDL3-associated asthma genotypes this work aims to target asthma susceptibility at a deeper level compared to the current anti-inflammatory and immunomodulatory strategies. Additional studies, assess the role of sphingolipid synthesis, in epithelial and immune cell host responses to viral respiratory infections, respiratory microbiome and vaccines.
Figure 1
Current Projects:
- Airway epithelial cell response to viral infections
- Sphingolipid synthesis regulators as respiratory vaccine adjuvants
- Sphingolipid metabolism in nasal and blood cells from children
Bio
Stefan Worgall earned his MD/PhD degree from Heidelberg University, Germany in medical sciences in 1992. He joined Cornell Medical School in 1995 as a postdoctoral fellow under the mentorship of Ronald Crystal. In 2004, he joined WCGS. He is now the Distinguished Professor of Pediatric Pulmonology at WCM and a member at WCGS.
Distinctions:
- Member, American Society of Clinical Investigation (2014)
- Klosterfrau Award, German Society for Pediatric Pulmonology (2015)
